Bronchopulmonary Dysplasia Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Bronchopulmonary Dysplasia Pipeline Drugs Market Overview
Bronchopulmonary dysplasia (BPD) can occur with multiple symptoms including tachypnea, tachycardia, increased respiratory effort (with retractions, nasal flaring, and grunting), and bluish skin color. The Treatment of BPD includes bronchodilators, diuretics, and antibiotics.
The Bronchopulmonary Dysplasia pipeline market research report provides an overview of the Bronchopulmonary Dysplasia pipeline landscape. The report provides comprehensive information on the therapeutics under development for Bronchopulmonary Dysplasia, complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA), and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history, and the latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Bronchopulmonary Dysplasia and features dormant and discontinued projects.
Bronchopulmonary Dysplasia Pipeline Drugs Market by Key Targets
Some of the targets of the Bronchopulmonary Dysplasia pipeline drugs market are Pulmonary Surfactant Associated Protein D, Retinoic Acid Receptor Alpha, Retinoic Acid Receptor Beta, Retinoic Acid Receptor Gamma, Thioredoxin, Apelin Receptor, C-X-C Chemokine Receptor Type 1, C-X-C Chemokine Receptor Type 2, Insulin Like Growth Factor 1 Receptor, and Insulin Like Growth Factor Binding Protein 3.
Bronchopulmonary Dysplasia Pipeline Drugs Market, by Targets
For more target insights, download a free report sample
Key Mechanisms of Action in the Bronchopulmonary Dysplasia Pipeline Drugs Market
Some of the key mechanisms of action of the Bronchopulmonary Dysplasia pipeline drugs market are Pulmonary Surfactant Associated Protein D Replacement, Retinoic Acid Receptor Alpha Agonist, Retinoic Acid Receptor Beta Agonist, Retinoic Acid Receptor Gamma Agonist, Thioredoxin Activator, Apelin Receptor Agonist, C-X-C Chemokine Receptor Type 1 Antagonist, C-X-C Chemokine Receptor Type 2 Antagonist, Insulin Like Growth Factor 1 Receptor Agonist, and Insulin Like Growth Factor Binding Protein 3 Inhibitor.
Bronchopulmonary Dysplasia Pipeline Drugs Market, by Mechanisms of Action
For more mechanisms of action insights, download a free report sample
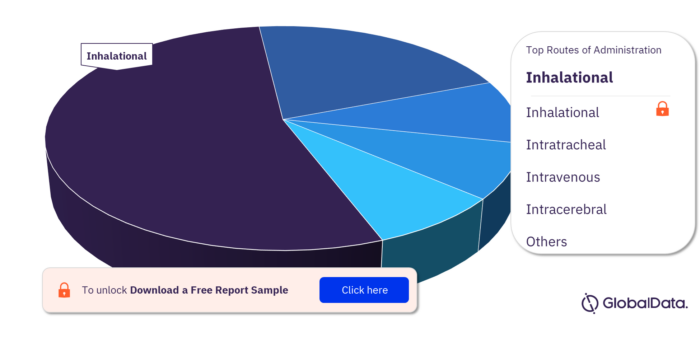
Key Routes of Administration in the Bronchopulmonary Dysplasia Pipeline Drugs Market
The key routes of administration in the Bronchopulmonary Dysplasia pipeline drugs market are inhalational, intratracheal, intravenous, intracerebral, oral, ophthalmic, and subcutaneous.
Bronchopulmonary Dysplasia Pipeline Drugs Market, by Routes of Administration
For more routes of administration insights, download a free report sample
Key Molecule Types in the Bronchopulmonary Dysplasia Pipeline Drugs Market
The molecule types in the Bronchopulmonary Dysplasia pipeline drugs market are small molecule, cell therapy, recombinant protein, antisense oligonucleotide, biologic, monoclonal antibody, and synthetic peptide.
Bronchopulmonary Dysplasia Pipeline Drugs Market, by Molecule Types
For more molecule type insights, download a free report sample
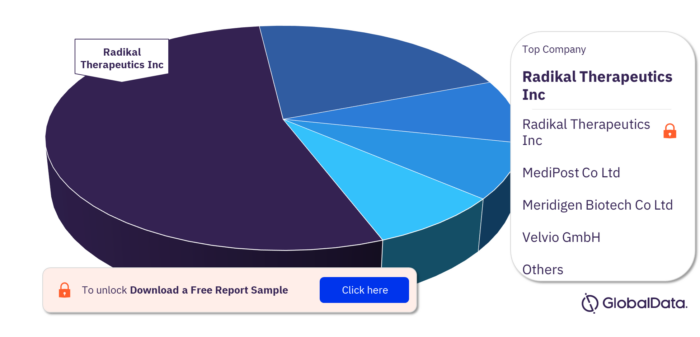
Key Bronchopulmonary Dysplasia Pipeline Drugs Market Companies
Some of the key companies in the Bronchopulmonary Dysplasia pipeline drugs market are Radikal Therapeutics Inc, MediPost Co Ltd, Meridigen Biotech Co Ltd, Velvio GmbH, Advent Therapeutics Inc, Airway Therapeutics Inc, APIE Therapeutics Inc, Ayuvis Research Inc, Boehringer Ingelheim International GmbH, and Chiesi Farmaceutici SpA. Radikal Therapeutics Inc has the highest pipeline products.
Radikal Therapeutics Inc: Radikal is a biotechnology company that discovers and develops transformative pharmaceuticals. The company develops organic nitrate-based nitric oxide donors, prodrug inhibitors of poly (ADP-ribose) polymerase-1, bifunctional potassium ATP-sensitive channel openers, stable biological reductants, and novel immunomodulators. Its pipeline products include R-107, R-190, R-197, R-554, R-801, R-807, and R-911. The company’s products are used for various clinical indications including peripheral arterial hypertension, adult respiratory distress syndrome, lung cancer, acute renal failure, radiation-induced fibrosis, acute lung injury, idiopathic pulmonary fibrosis, multiple sclerosis, sepsis, and others. Radikal Therapeutics is headquartered in West Tisbury, Massachusetts, the US.
Bronchopulmonary Dysplasia Pipeline Drugs Market, by Key Companies
To know more about key companies, download a free report sample
Market Report Overview
| Key Targets | Pulmonary Surfactant Associated Protein D, Retinoic Acid Receptor Alpha, Retinoic Acid Receptor Beta, Retinoic Acid Receptor Gamma, Thioredoxin, Apelin Receptor, C-X-C Chemokine Receptor Type 1, C-X-C Chemokine Receptor Type 2, Insulin Like Growth Factor 1 Receptor, and Insulin Like Growth Factor Binding Protein 3 |
| Key Mechanisms of Action | Pulmonary Surfactant Associated Protein D Replacement, Retinoic Acid Receptor Alpha Agonist, Retinoic Acid Receptor Beta Agonist, Retinoic Acid Receptor Gamma Agonist, Thioredoxin Activator, Apelin Receptor Agonist, C-X-C Chemokine Receptor Type 1 Antagonist, C-X-C Chemokine Receptor Type 2 Antagonist, Insulin Like Growth Factor 1 Receptor Agonist, and Insulin Like Growth Factor Binding Protein 3 Inhibitor |
| Key Routes of Administration | Inhalational, Intratracheal, Intravenous, Intracerebral, Oral, Ophthalmic, and Subcutaneous |
| Key Molecule Types | Small Molecule, Cell Therapy, Recombinant Protein, Antisense Oligonucleotide, Biologic, Monoclonal Antibody, and Synthetic Peptide |
| Key Companies | Radikal Therapeutics Inc, MediPost Co Ltd, Meridigen Biotech Co Ltd, Velvio GmbH, Advent Therapeutics Inc, Airway Therapeutics Inc, APIE Therapeutics Inc, Ayuvis Research Inc, Boehringer Ingelheim International GmbH, and Chiesi Farmaceutici SpA |
Scope
This report provides:
- A snapshot of the global therapeutic landscape of Bronchopulmonary Dysplasia (Respiratory).
- Reviews of pipeline therapeutics for Bronchopulmonary Dysplasia (Respiratory) by companies and universities/research institutes based on information derived from company and industry-specific sources.
- Pipeline products based on several stages of development ranging from pre-registration to discovery and undisclosed stages.
- Descriptive drug profiles for the pipeline products which include product description, descriptive MoA, R&D brief, licensing and collaboration details & other developmental activities.
- Reviews of key companies involved in Bronchopulmonary Dysplasia (Respiratory) therapeutics and enlists all their major and minor projects.
- Evaluation of Bronchopulmonary Dysplasia (Respiratory) therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA) and molecule type.
- All the dormant and discontinued pipeline projects.
- Reviews of the latest news and deals related to pipeline therapeutics for Bronchopulmonary Dysplasia (Respiratory).
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Bronchopulmonary Dysplasia (Respiratory).
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and it’s most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Bronchopulmonary Dysplasia (Respiratory) pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from pipeline.
Airway Therapeutics Inc
APIE Therapeutics Inc
Ayuvis Research Inc
Boehringer Ingelheim International GmbH
Chiesi Farmaceutici SpA
Exo Biologics SA
Insmed Inc
MediPost Co Ltd
Meridigen Biotech Co Ltd
Orphanix GmbH
Radikal Therapeutics Inc
Syntrix Pharmaceuticals
Takeda Pharmaceutical Co Ltd
The Cell Factory BVBA
Trimunocor Ltd
Velvio GmbH
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets of the Bronchopulmonary Dysplasia pipeline drugs market?
Some of the targets of the Bronchopulmonary Dysplasia pipeline drugs market are Pulmonary Surfactant Associated Protein D, Retinoic Acid Receptor Alpha, Retinoic Acid Receptor Beta, Retinoic Acid Receptor Gamma, Thioredoxin, Apelin Receptor, C-X-C Chemokine Receptor Type 1, C-X-C Chemokine Receptor Type 2, Insulin Like Growth Factor 1 Receptor, and Insulin Like Growth Factor Binding Protein 3.
-
What are the key mechanisms of action of the Bronchopulmonary Dysplasia pipeline drugs market?
Some of the mechanisms of action of the Bronchopulmonary Dysplasia pipeline drugs market are Pulmonary Surfactant Associated Protein D Replacement, Retinoic Acid Receptor Alpha Agonist, Retinoic Acid Receptor Beta Agonist, Retinoic Acid Receptor Gamma Agonist, Thioredoxin Activator, Apelin Receptor Agonist, C-X-C Chemokine Receptor Type 1 Antagonist, C-X-C Chemokine Receptor Type 2 Antagonist, Insulin Like Growth Factor 1 Receptor Agonist, and Insulin Like Growth Factor Binding Protein 3 Inhibitor.
-
What are the routes of administration in the Bronchopulmonary Dysplasia pipeline drugs market?
The routes of administration in the Bronchopulmonary Dysplasia pipeline drugs market are inhalational, intratracheal, intravenous, intracerebral, oral, ophthalmic, and subcutaneous.
-
What are the molecule types in the Bronchopulmonary Dysplasia pipeline drugs market?
The molecule types in the Bronchopulmonary Dysplasia pipeline drugs market are small molecule, cell therapy, recombinant protein, antisense oligonucleotide, biologic, monoclonal antibody, and synthetic peptide.
-
Which are the key companies in the Bronchopulmonary Dysplasia pipeline drugs market?
Some of the key companies in the Bronchopulmonary Dysplasia pipeline drugs market are Radikal Therapeutics Inc, MediPost Co Ltd, Meridigen Biotech Co Ltd, Velvio GmbH, Advent Therapeutics Inc, Airway Therapeutics Inc, APIE Therapeutics Inc, Ayuvis Research Inc, Boehringer Ingelheim International GmbH, and Chiesi Farmaceutici SpA.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Respiratory reports