Clinical Decision Support Systems – Pipeline Products by Stage of Development 37
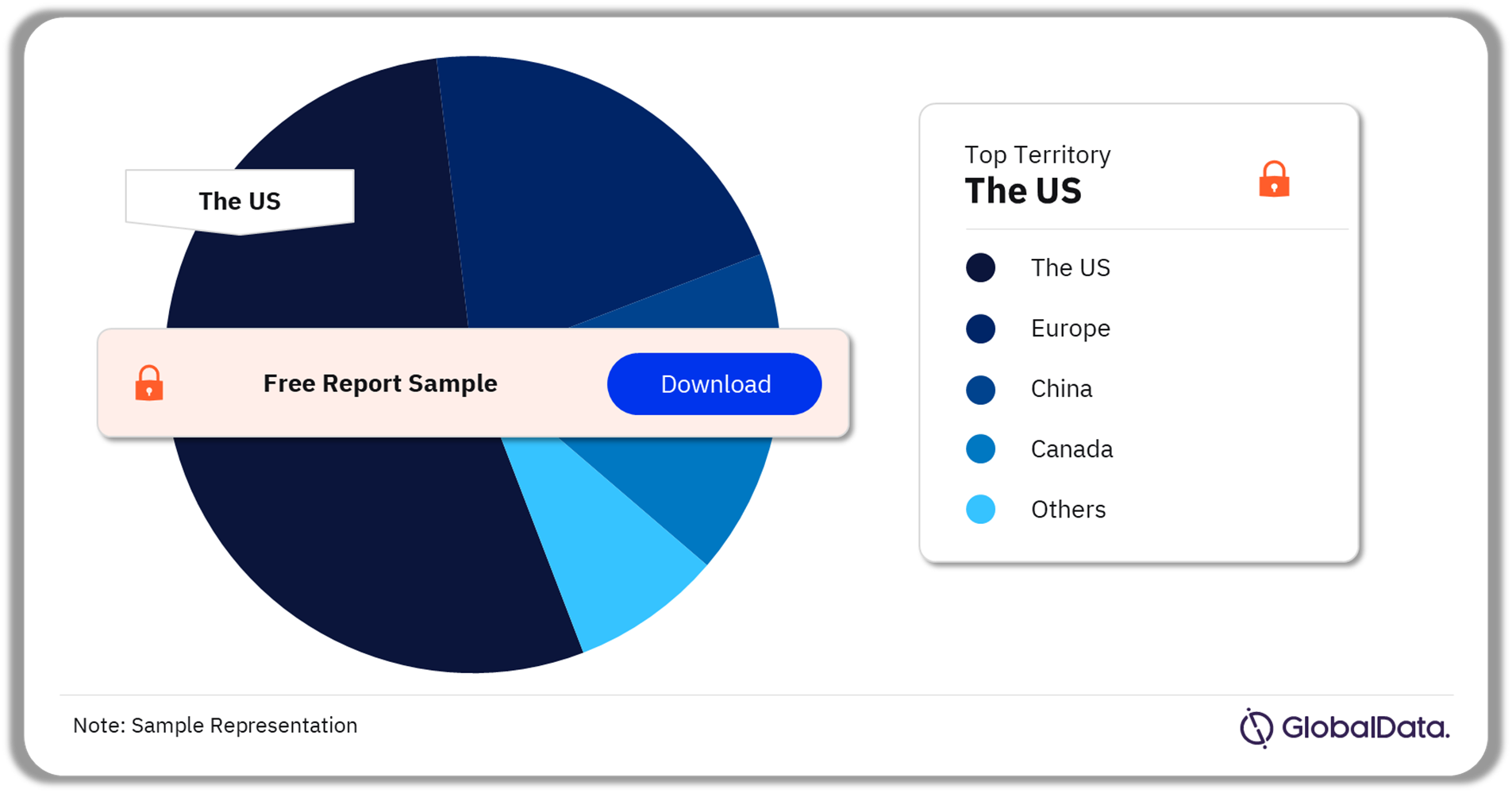
Clinical Decision Support Systems – Pipeline Products by Territory 38
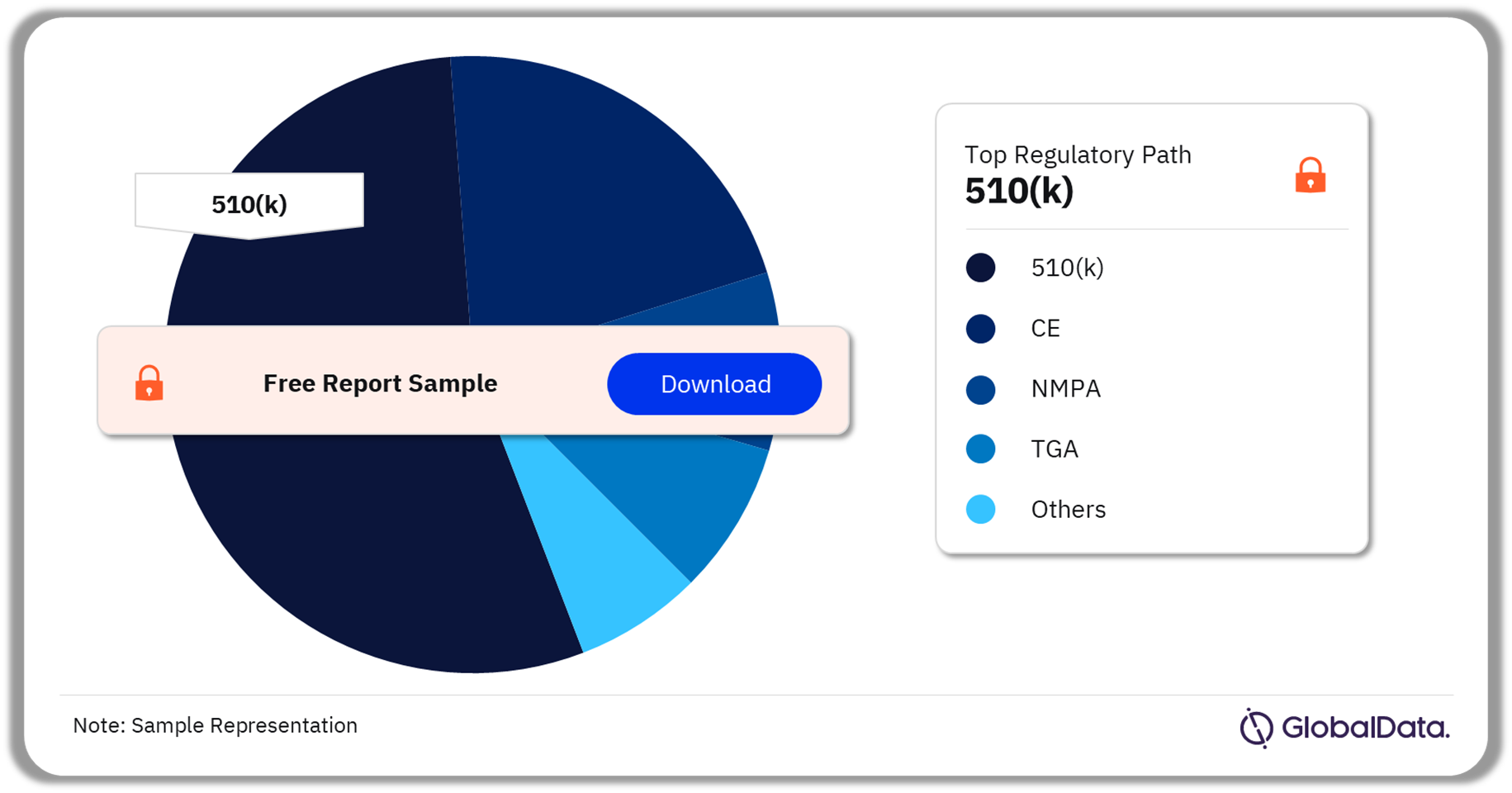
Clinical Decision Support Systems – Pipeline Products by Regulatory Path 40
Clinical Decision Support Systems – Pipeline Products by Estimated Approval Date 41
Clinical Decision Support Systems – Ongoing Clinical Trials 42
Clinical Decision Support Systems Companies – Pipeline Products by Stage of Development 43
Clinical Decision Support Systems – Pipeline Products by Stage of Development 53
Aalborg University Pipeline Products & Ongoing Clinical Trials Overview 63
GLUCOSAFE 2 Software – Product Status 63
GLUCOSAFE 2 Software – Product Description 63
Aalborg University – Ongoing Clinical Trials Overview 64
GLUCOSAFE 2 Software – GLUCOSAFE 2 – a New Tool for Nutritional Management and Insulin-therapy in the Intensive Care Unit: a Pilot Randomized Controlled Trial 65
Abiomed Inc Pipeline Products & Ongoing Clinical Trials Overview 66
Artificial Intelligence Algorithm – Product Status 66
Artificial Intelligence Algorithm – Product Description 66
Aclarion Inc Pipeline Products & Ongoing Clinical Trials Overview 67
Nociscan – Product Status 67
Nociscan – Product Description 67
Aclarion Inc – Ongoing Clinical Trials Overview 69
Nociscan – Clinical Evaluation of NOCISCAN-lumbar Spine (LS) Disc MR Spectroscopy (MRS) for Diagnosis of Discogenic Low Back Pain and Correlation with Surgical Outcomes 70
Nociscan – The BEST Trial: Biomarkers for Evaluating Spine Treatments 70
Adaptive Technology Consulting LLC Pipeline Products & Ongoing Clinical Trials Overview 71
Paisley – Product Status 71
Paisley – Product Description 71
Admera Health LLC Pipeline Products & Ongoing Clinical Trials Overview 72
AGIS Genomics Platform – Nutrigenomics Module – Product Status 72
AGIS Genomics Platform – Nutrigenomics Module – Product Description 72
Advinow Inc Pipeline Products & Ongoing Clinical Trials Overview 73
Automated Healthcare System – Product Status 73
Automated Healthcare System – Product Description 73
AHEPA University Hospital Pipeline Products & Ongoing Clinical Trials Overview 74
IcaDx (Interpretable Computer-Aided Diagnosis) System – Product Status 74
IcaDx (Interpretable Computer-Aided Diagnosis) System – Product Description 74
AI Medical Technology AB Pipeline Products & Ongoing Clinical Trials Overview 75
Dermalyzer – Product Status 75
Dermalyzer – Product Description 75
ai4gi Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 76
AI Based Colon Cancer Screening – Product Status 76
AI Based Colon Cancer Screening – Product Description 76
AI4medicine Pipeline Products & Ongoing Clinical Trials Overview 77
Decision Support System – Acute Stroke – Product Status 77
Decision Support System – Acute Stroke – Product Description 77
Ai-Assisted Hematologic Analytic and Decision Support Pipeline Products & Ongoing Clinical Trials Overview 78
AI-Enabled Clinical Decision Support Tool – Blood Cancer – Product Status 78
AI-Enabled Clinical Decision Support Tool – Blood Cancer – Product Description 78
Aidoc Ltd Pipeline Products & Ongoing Clinical Trials Overview 79
AI-Based Decision Support Software – Abdomen – Product Status 79
AI-Based Decision Support Software – Abdomen – Product Description 79
AI-Based Decision Support Software – Chest – Product Status 79
AI-Based Decision Support Software – Chest – Product Description 80
Aifred Health Inc Pipeline Products & Ongoing Clinical Trials Overview 81
Clinical Decision Support – Mental Health – Product Status 81
Clinical Decision Support – Mental Health – Product Description 81
Aifred Health Inc – Ongoing Clinical Trials Overview 82
Clinical Decision Support – Mental Health – Artificial Intelligence in Depression – Medication Enhancement: A Randomized, Patient and Rater Blinded, Active-controlled Trial of a Hybrid-Classic/Machine-learning Enabled Clinical Decision Aid for Personalized and Individualized Pharmacological Depression Treatment Selection 83
Aignostics GmbH Pipeline Products & Ongoing Clinical Trials Overview 84
Digital Imaging Analysis System – Product Status 84
Digital Imaging Analysis System – Product Description 84
AirStrip Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 85
Clinical Decision Support Tool – Critical Care – Product Status 85
Clinical Decision Support Tool – Critical Care – Product Description 85
Clinical Decision Support Tool – Heart Diseases – Product Status 85
Clinical Decision Support Tool – Heart Diseases – Product Description 86
Mobile Acute Care Early Warning System (mACEWS) – Product Status 86
Mobile Acute Care Early Warning System (mACEWS) – Product Description 86
Alan Turing Institute Almere Pipeline Products & Ongoing Clinical Trials Overview 88
Machine Learning Algorithm – Cystic Fibrosis – Product Status 88
Machine Learning Algorithm – Cystic Fibrosis – Product Description 88
AlertWatch Inc Pipeline Products & Ongoing Clinical Trials Overview 89
AlertWatch:ICU – Product Status 89
AlertWatch:ICU – Product Description 89
AlertWatch:PACU – Product Status 90
AlertWatch:PACU – Product Description 90
AlertWatch Inc – Ongoing Clinical Trials Overview 91
AlertWatch:ICU – Telemedicine Notifications with Machine Learning for Postoperative Care 92
AlgorithmRx, LLC Pipeline Products & Ongoing Clinical Trials Overview 93
aRx Statin Advisor – Product Status 93
aRx Statin Advisor – Product Description 93
All India Institute of Medical Sciences Pipeline Products & Ongoing Clinical Trials Overview 94
I-TREC (Integrated Tracking, Referral, Electronic Decision Support, and Care Coordination) System – Product Status 94
I-TREC (Integrated Tracking, Referral, Electronic Decision Support, and Care Coordination) System – Product Description 94
Allegheny-Singer Research Institute Pipeline Products & Ongoing Clinical Trials Overview 95
PHORA – Product Status 95
PHORA – Product Description 95
American College of Rheumatology Pipeline Products & Ongoing Clinical Trials Overview 96
Clinical Decision Support Tool – Axial Spondyloarthritis – Product Status 96
Clinical Decision Support Tool – Axial Spondyloarthritis – Product Description 96
Ampel BioSolutions LLC Pipeline Products & Ongoing Clinical Trials Overview 97
CardioGENE – Product Status 97
CardioGENE – Product Description 97
CovGENE – Product Status 98
CovGENE – Product Description 98
DermaGENE – Product Status 98
DermaGENE – Product Description 98
Analytic Diabetic Systems, LLC Pipeline Products & Ongoing Clinical Trials Overview 100
Blood Sugar Monitoring Software – Product Status 100
Blood Sugar Monitoring Software – Product Description 100
Anumana Inc Pipeline Products & Ongoing Clinical Trials Overview 101
ECG AI Algorithm – Aortic Stenosis – Product Status 101
ECG AI Algorithm – Aortic Stenosis – Product Description 101
ECG AI Algorithm – Atherosclerosis – Product Status 102
ECG AI Algorithm – Atherosclerosis – Product Description 102
ECG AI Algorithm – Atrial Fibrillation In Normal Sinus Rhythm – Product Status 103
ECG AI Algorithm – Atrial Fibrillation In Normal Sinus Rhythm – Product Description 103
ECG AI Algorithm – Hyperkalemia – Product Status 103
ECG AI Algorithm – Hyperkalemia – Product Description 103
ECG AI Algorithm – Hypertrophic Cardiomyopathy – Product Status 104
ECG AI Algorithm – Hypertrophic Cardiomyopathy – Product Description 104
ECG AI Algorithm – Myocarditis – Product Status 105
ECG AI Algorithm – Myocarditis – Product Description 105
ECG AI Algorithm – Pulmonary Hypertension – Product Status 105
ECG AI Algorithm – Pulmonary Hypertension – Product Description 106
Low Ejection Fraction AI-ECG Algorithm – Product Status 106
Low Ejection Fraction AI-ECG Algorithm – Product Description 106
Anumana Inc – Ongoing Clinical Trials Overview 108
Low Ejection Fraction AI-ECG Algorithm – A Prospective Pragmatic Cluster-Randomized Care-as-Usual Controlled Study to Evaluate the Impact of an ECG-Based AI Algorithm to Detect Low Left Ventricular Ejection Fraction on Diagnosis Rates of LVEF =40% in the Outpatient Setting 109
Apollo Medical Imaging Technology Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 110
AI-Based Clinical Decision Support Software – Product Status 110
AI-Based Clinical Decision Support Software – Product Description 110
Arcturis Data Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
SENSE System – Product Status 111
SENSE System – Product Description 111
SYNE-COV – Product Status 111
SYNE-COV – Product Description 112
Arne BV Pipeline Products & Ongoing Clinical Trials Overview 113
Neonatal Care Algorithm – Product Status 113
Neonatal Care Algorithm – Product Description 113
Atomo Inc Pipeline Products & Ongoing Clinical Trials Overview 114
PERCEPTION – Product Status 114
PERCEPTION – Product Description 114
Auckland University of Technology Pipeline Products & Ongoing Clinical Trials Overview 115
Medical Decision Support System – Product Status 115
Medical Decision Support System – Product Description 115
Automated Reliable Tissue Diagnostics AG Pipeline Products & Ongoing Clinical Trials Overview 116
ArtidisNet – Product Status 116
ArtidisNet – Product Description 116
Automated Reliable Tissue Diagnostics AG – Ongoing Clinical Trials Overview 117
ArtidisNet – Trial Of PreoperAtive Radiation (TOPAz): A Randomized Trial Comparing Hypofractionated Versus Conventionally Fractionated Preoperative Radiation Followed by Mastectomy With Immediate Autologous Breast Reconstruction With Integrated Nanomechanical Biomarker Evaluation 118
Automedics Medical Systems Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 119
HepGuide Decision Support Software – Product Status 119
HepGuide Decision Support Software – Product Description 119
WarfGuide Decision Support Software – Product Status 119
WarfGuide Decision Support Software – Product Description 120
Autonomous Healthcare Inc Pipeline Products & Ongoing Clinical Trials Overview 121
E-Fusion – Product Status 121
E-Fusion – Product Description 121
Bausch & Lomb Inc Pipeline Products & Ongoing Clinical Trials Overview 122
Next-Generation Eyetelligence – Product Status 122
Next-Generation Eyetelligence – Product Description 122
Bayer AG Pipeline Products & Ongoing Clinical Trials Overview 123
AI Software – Chronic Thromboembolic Pulmonary Hypertension – Product Status 123
AI Software – Chronic Thromboembolic Pulmonary Hypertension – Product Description 123
Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 124
LvTrack – Product Status 124
LvTrack – Product Description 124
Berg LLC Pipeline Products & Ongoing Clinical Trials Overview 125
Medication Adherence Algorithm – Product Status 125
Medication Adherence Algorithm – Product Description 125
bioMerieux Inc Pipeline Products & Ongoing Clinical Trials Overview 126
Clinical Digital Solution – Product Status 126
Clinical Digital Solution – Product Description 126
BioSensics LLC Pipeline Products & Ongoing Clinical Trials Overview 127
Software Tool – Musculoskeletal Tumours – Product Status 127
Software Tool – Musculoskeletal Tumours – Product Description 127
Blenderhouse Pipeline Products & Ongoing Clinical Trials Overview 128
Cardiac Health Risk Stratification System – Product Status 128
Cardiac Health Risk Stratification System – Product Description 128
Blue Eye Soft Corp Pipeline Products & Ongoing Clinical Trials Overview 129
BlueDocAI – Product Status 129
BlueDocAI – Product Description 129
Brain Electrophysiology Laboratory LLC Pipeline Products & Ongoing Clinical Trials Overview 130
EEG Imagining Decision Support Software – Product Status 130
EEG Imagining Decision Support Software – Product Description 130
Braingaze SL Pipeline Products & Ongoing Clinical Trials Overview 131
ADHD Home Therapy – Product Status 131
ADHD Home Therapy – Product Description 131
ADHD Online Risk Check – Product Status 131
ADHD Online Risk Check – Product Description 132
Alzheimer’s/MCI Home Therapy – Product Status 132
Alzheimer’s/MCI Home Therapy – Product Description 132
Brainomix Ltd Pipeline Products & Ongoing Clinical Trials Overview 133
e-ACT Software – Product Status 133
e-ACT Software – Product Description 133
BrightOutcome Inc. Pipeline Products & Ongoing Clinical Trials Overview 134
AAME (Alcohol Abuse Management Environment (AAME) System – Product Status 134
AAME (Alcohol Abuse Management Environment (AAME) System – Product Description 134
CancerCostDetox – Product Status 135
CancerCostDetox – Product Description 135
myCare2 – Product Status 135
myCare2 – Product Description 135
Personalized Online Weight and Exercise Response System (POWERS) – Product Status 136
Personalized Online Weight and Exercise Response System (POWERS) – Product Description 136
Cardiogram Inc Pipeline Products & Ongoing Clinical Trials Overview 137
Cardiogram Heart Health App – Postural Orthostatic Tachycardia Syndrome (POTS) – Product Status 137
Cardiogram Heart Health App – Postural Orthostatic Tachycardia Syndrome (POTS) – Product Description 137
CareDx Inc Pipeline Products & Ongoing Clinical Trials Overview 138
AiKidney – Product Status 138
AiKidney – Product Description 138
Clinical Decision Support Tool – Product Status 139
Clinical Decision Support Tool – Product Description 139
Carevive Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 140
Electronic Interoperable CDS Tool – Product Status 140
Electronic Interoperable CDS Tool – Product Description 140
Carnegie Mellon University Pipeline Products & Ongoing Clinical Trials Overview 141
Cardiac Outcomes Risk Assessment – Heart Failure – Product Status 141
Cardiac Outcomes Risk Assessment – Heart Failure – Product Description 141
Case Western Reserve University Pipeline Products & Ongoing Clinical Trials Overview 142
LunIOTx – Product Status 142
LunIOTx – Product Description 142
Cellnovo Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 143
PEPPER (Patient Empowerment Through Predictive Personalised Decision Support) System – Product Status 143
PEPPER (Patient Empowerment Through Predictive Personalised Decision Support) System – Product Description 143
Children’s Hospital of Philadelphia Pipeline Products & Ongoing Clinical Trials Overview 144
Machine Learning Software Tool – Sepsis – Product Status 144
Machine Learning Software Tool – Sepsis – Product Description 144
Children’s Research Institute Pipeline Products & Ongoing Clinical Trials Overview 145
Quantitative Imaging Tool – Craniosynostosis – Product Status 145
Quantitative Imaging Tool – Craniosynostosis – Product Description 145
Coapt LLC Pipeline Products & Ongoing Clinical Trials Overview 146
Connected Health System – Product Status 146
Connected Health System – Product Description 146
Cohere Health Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 147
CohereNext – Product Status 147
CohereNext – Product Description 147
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 148
Decision Support Tool – Breast Cancer – Product Status 148
Decision Support Tool – Breast Cancer – Product Description 148
Combinostics Oy Pipeline Products & Ongoing Clinical Trials Overview 149
cNeuro cARIA – Product Status 149
cNeuro cARIA – Product Description 149
cNeuro cPET – Product Status 149
cNeuro cPET – Product Description 150
Commonwealth Scientific and Industrial Research Organisation Pipeline Products & Ongoing Clinical Trials Overview 151
AssessCP – Product Status 151
AssessCP – Product Description 151
Computer Technology Associates Inc Pipeline Products & Ongoing Clinical Trials Overview 152
Clinical Decision Support System – Pediatric Severe Sepsis – Product Status 152
Clinical Decision Support System – Pediatric Severe Sepsis – Product Description 152
Computer Technology Associates Inc – Ongoing Clinical Trials Overview 153
Clinical Decision Support System – Pediatric Severe Sepsis – Biomarker-enhanced Artificial Intelligence Based Pediatric Sepsis Screening Tool Towards Early Recognition and Personalized Therapeutics 154
ContextVision AB Pipeline Products & Ongoing Clinical Trials Overview 155
DST – Breast Cancer – Product Status 155
DST – Breast Cancer – Product Description 155
DST – Lung Cancer – Product Status 156
DST – Lung Cancer – Product Description 156
Continuous Precision Medicine Inc Pipeline Products & Ongoing Clinical Trials Overview 157
CPMRx – Product Status 157
CPMRx – Product Description 157
Corin Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 158
OPSReView – Product Status 158
OPSReView – Product Description 158
CorticoMetrics LLC Pipeline Products & Ongoing Clinical Trials Overview 159
Clinical Decision Support Software – Product Status 159
Clinical Decision Support Software – Product Description 159
Crosscope Inc Pipeline Products & Ongoing Clinical Trials Overview 160
CrosscopeDx – Liver Cancer – Product Status 160
CrosscopeDx – Liver Cancer – Product Description 160
Curemetrix Inc Pipeline Products & Ongoing Clinical Trials Overview 161
cmDensity – Product Status 161
cmDensity – Product Description 161
Dana-Farber Cancer Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 162
AI Based Muscle Mass Assessment Tool – Product Status 162
AI Based Muscle Mass Assessment Tool – Product Description 162
Dascena Inc Pipeline Products & Ongoing Clinical Trials Overview 163
HindSight – Product Status 163
HindSight – Product Description 163
Dascena Inc – Ongoing Clinical Trials Overview 164
HindSight – Evaluation of the Efficacy of HindSight Algorithm to Predict the Onset of Sepsis in Patients 165
Daxor Corp Pipeline Products & Ongoing Clinical Trials Overview 166
Clinical Decision Support Software – Product Status 166
Clinical Decision Support Software – Product Description 166
Sepsis FLO (Sepsis Fluid Optimizer) – Product Status 166
Sepsis FLO (Sepsis Fluid Optimizer) – Product Description 167
Deontics Ltd Pipeline Products & Ongoing Clinical Trials Overview 168
PROSAIC-DS (PROState AI in Cancer – Decision Support) – Product Status 168
PROSAIC-DS (PROState AI in Cancer – Decision Support) – Product Description 168
Deontics Ltd – Ongoing Clinical Trials Overview 169
PROSAIC-DS (PROState AI in Cancer – Decision Support) – PROSAIC-DS (PROState AI in Cancer – Decision Support): Evaluation of the Deontics AI Platform for Evidence-based Treatment Planning in Multidisciplinary Cancer Care: Increasing Compliance and Streamlining MDTs in Prostate Cancer 170
DermAb.io Pipeline Products & Ongoing Clinical Trials Overview 171
Clinical Decision Support System – Product Status 171
Clinical Decision Support System – Product Description 171
Diag-Nose Medical Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 172
Clinical Decision Support System – Chronic Rhinosinusitis – Product Status 172
Clinical Decision Support System – Chronic Rhinosinusitis – Product Description 172
Directed Systems Ltd Pipeline Products & Ongoing Clinical Trials Overview 173
Hypotension Decision Assist – Product Status 173
Hypotension Decision Assist – Product Description 173
Doctrin AB Pipeline Products & Ongoing Clinical Trials Overview 174
Decision Support Tool – Product Status 174
Decision Support Tool – Product Description 174
Dokkyo Medical University Pipeline Products & Ongoing Clinical Trials Overview 175
Clinical Decision Support System – Auscultation – Product Status 175
Clinical Decision Support System – Auscultation – Product Description 175
Dokkyo Medical University – Ongoing Clinical Trials Overview 176
Clinical Decision Support System – Auscultation – The Utility of Clinical Decision Support from Machine Learning Model for Auscultation: Open-Label Randomized Controlled Pilot Trial 177
DSX Therapeutics LLC Pipeline Products & Ongoing Clinical Trials Overview 178
Expertcare – Hypertension – Product Status 178
Expertcare – Hypertension – Product Description 178
Eko Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 179
Clinical Decision Support Algorithm – Arteriovenous Fitsula – Product Status 179
Clinical Decision Support Algorithm – Arteriovenous Fitsula – Product Description 179
Clinical Decision Support Algorithm – Pulmonary Hypertension – Product Status 180
Clinical Decision Support Algorithm – Pulmonary Hypertension – Product Description 180
Screening Algorithm – Aortic Stenosis – Product Status 180
Screening Algorithm – Aortic Stenosis – Product Description 180
Embryonics Ltd Pipeline Products & Ongoing Clinical Trials Overview 182
Ubar – Product Status 182
Ubar – Product Description 182
Encompass Health Corp Pipeline Products & Ongoing Clinical Trials Overview 183
Clinical Decision Support Tool – Product Status 183
Clinical Decision Support Tool – Product Description 183
Ensofy Inc Pipeline Products & Ongoing Clinical Trials Overview 184
VoiceAI – Product Status 184
VoiceAI – Product Description 184
eNursing LLC Pipeline Products & Ongoing Clinical Trials Overview 185
PAINConsultN – Product Status 185
PAINConsultN – Product Description 185
Eodyne Systems SL Pipeline Products & Ongoing Clinical Trials Overview 186
Clinical Decision Support Tool – Neurodegenerative Disease – Product Status 186
Clinical Decision Support Tool – Neurodegenerative Disease – Product Description 186
Esco Lifesciences Group Pipeline Products & Ongoing Clinical Trials Overview 187
Decision Support Tool – MIRI TL – Product Status 187
Decision Support Tool – MIRI TL – Product Description 187
Experiad LLC Pipeline Products & Ongoing Clinical Trials Overview 188
Acuity – Product Status 188
Acuity – Product Description 188
Eyoto Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 189
Diagnosis Support System – Product Status 189
Diagnosis Support System – Product Description 189
Fairtility Pipeline Products & Ongoing Clinical Trials Overview 190
CHLOE EQ (Cultivating Human Life through Optimal Embryos) – Product Status 190
CHLOE EQ (Cultivating Human Life through Optimal Embryos) – Product Description 190
FIGUR8 Inc Pipeline Products & Ongoing Clinical Trials Overview 191
Musculoskeletal Diagnostic System – Knee Injury – Product Status 191
Musculoskeletal Diagnostic System – Knee Injury – Product Description 191
Musculoskeletal Diagnostic System – Lower Back Injury – Product Status 192
Musculoskeletal Diagnostic System – Lower Back Injury – Product Description 192
Musculoskeletal Diagnostic System – Lumbar Spine Injury – Product Status 192
Musculoskeletal Diagnostic System – Lumbar Spine Injury – Product Description 193
Musculoskeletal Diagnostic System – Neck Injury – Product Status 193
Musculoskeletal Diagnostic System – Neck Injury – Product Description 193
FlowView Diagnostics BV Pipeline Products & Ongoing Clinical Trials Overview 195
FlowView Eclipse – Product Status 195
FlowView Eclipse – Product Description 195
GE HealthCare Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 196
Brain Mobile App – Product Status 196
Brain Mobile App – Product Description 196
CardioVisio – Atrial Fibrillation – Product Status 197
CardioVisio – Atrial Fibrillation – Product Description 197
General Hospital of the People’s Liberation Army Pipeline Products & Ongoing Clinical Trials Overview 198
Diagnostic Assistance Decision Support System – Product Status 198
Diagnostic Assistance Decision Support System – Product Description 198
General Hospital of the People’s Liberation Army – Ongoing Clinical Trials Overview 199
Diagnostic Assistance Decision Support System – Research and Development of Diagnostic Assistance Decision Support System for Novel Coronavirus Pneumonia (COVID-19) Based on Big Data Technology 200
Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 201
Organ Transplant Decision Tool – Product Status 201
Organ Transplant Decision Tool – Product Description 201
Griffith University Pipeline Products & Ongoing Clinical Trials Overview 202
The Digital Athlete – Product Status 202
The Digital Athlete – Product Description 202
Guerbet SA Pipeline Products & Ongoing Clinical Trials Overview 203
AI Based Diagnostic Tool – Liver Cancer – Product Status 203
AI Based Diagnostic Tool – Liver Cancer – Product Description 203
Watson Imaging Care Advisor System – Product Status 204
Watson Imaging Care Advisor System – Product Description 204
H. Lee Moffitt Cancer Center & Research Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 205
Decision Support Tool – Product Status 205
Decision Support Tool – Product Description 205
Decision Support Tool – Oncology – Product Status 206
Decision Support Tool – Oncology – Product Description 206
Harvard Medical School Pipeline Products & Ongoing Clinical Trials Overview 207
CHARM – Product Status 207
CHARM – Product Description 207
Healthplus.ai BV Pipeline Products & Ongoing Clinical Trials Overview 208
Periscope – Product Status 208
Periscope – Product Description 208
HealthTech Solutions LLC Pipeline Products & Ongoing Clinical Trials Overview 209
Clinical Decision Support Software – Product Status 209
Clinical Decision Support Software – Product Description 209
Hemeo BV Pipeline Products & Ongoing Clinical Trials Overview 210
Hemeo – Product Status 210
Hemeo – Product Description 210
Henry Ford Health System Pipeline Products & Ongoing Clinical Trials Overview 211
Decision Support System – Epilepsy – Product Status 211
Decision Support System – Epilepsy – Product Description 211
Hera MI Pipeline Products & Ongoing Clinical Trials Overview 212
Breast-SlimView – Product Status 212
Breast-SlimView – Product Description 212
Heuron Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 213
BT-M01 – Product Status 213
BT-M01 – Product Description 213
Heuron Co Ltd – Ongoing Clinical Trials Overview 214
BT-M01 – An Open Label, Single-Center and Random-Selection Retrospective Pivotal Study of Clinical Decision Support System for Brain Metastasis Using Brain MR Images 215
Hitachi Ltd Pipeline Products & Ongoing Clinical Trials Overview 216
Pharmacotherapy Selection System – Product Status 216
Pharmacotherapy Selection System – Product Description 216
Hospital del Mar Pipeline Products & Ongoing Clinical Trials Overview 217
A-BIRTHPERFORM Digital Tool – Product Status 217
A-BIRTHPERFORM Digital Tool – Product Description 217
Idoven 1903 SL Pipeline Products & Ongoing Clinical Trials Overview 218
Willem AI Based Clinical Decision Support Software – Product Status 218
Willem AI Based Clinical Decision Support Software – Product Description 218
Idoven 1903 SL – Ongoing Clinical Trials Overview 219
Willem AI Based Clinical Decision Support Software – Evaluation of Electrocardiographic Data From High-risk Cardiac Patients Using Willem Cardiologist-level Artificial Intelligence Software WILLEM Trial 220
Willem AI Based Clinical Decision Support Software – LESTONNAC Study: Observed ST Elevation Does Not Require Acute Cardiac Necrosis 220
iHealthLabs Europe Pipeline Products & Ongoing Clinical Trials Overview 221
POWER2DM System – Product Status 221
POWER2DM System – Product Description 221
Ilex Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 222
eL@b – Product Status 222
eL@b – Product Description 222
Illumigyn Ltd Pipeline Products & Ongoing Clinical Trials Overview 223
AI Clinical Decision Support Software – Product Status 223
AI Clinical Decision Support Software – Product Description 223
Imaxdi. Pipeline Products & Ongoing Clinical Trials Overview 224
ISHEM Intelligent System – Product Status 224
ISHEM Intelligent System – Product Description 224
Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 225
AI Based Diagnosis – Small Vessel Disease – Product Status 225
AI Based Diagnosis – Small Vessel Disease – Product Description 225
AI Software – Cardiac Rhythm Devices – Product Status 226
AI Software – Cardiac Rhythm Devices – Product Description 226
Increase Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 227
Clinical Decision Support System – Product Status 227
Clinical Decision Support System – Product Description 227
InformAI LLC Pipeline Products & Ongoing Clinical Trials Overview 228
AI Based Patient Outcome Predictor – Product Status 228
AI Based Patient Outcome Predictor – Product Description 228
AI Based Surgical Risk Predictor – Product Status 229
AI Based Surgical Risk Predictor – Product Description 229
Predictor AI Tool – Liver Transplant – Product Status 229
Predictor AI Tool – Liver Transplant – Product Description 229
InfotechSoft Inc Pipeline Products & Ongoing Clinical Trials Overview 231
Deep-CDS – Product Status 231
Deep-CDS – Product Description 231
Inpro medical LLC Pipeline Products & Ongoing Clinical Trials Overview 232
Decision Support Software – Product Status 232
Decision Support Software – Product Description 232
InsightRX Inc Pipeline Products & Ongoing Clinical Trials Overview 233
Clinical Decision Support Tool – Diabetes Management – Product Status 233
Clinical Decision Support Tool – Diabetes Management – Product Description 233
Clinical Decision Support Tool – Oncology – Product Status 233
Clinical Decision Support Tool – Oncology – Product Description 234
Clinical Decision Support Tool – Ophthalmology – Product Status 234
Clinical Decision Support Tool – Ophthalmology – Product Description 234
InsightRX PGx – Product Status 235
InsightRX PGx – Product Description 235
Intelligent Medical Objects Inc Pipeline Products & Ongoing Clinical Trials Overview 236
Clinical Decision Support Tool – Product Status 236
Clinical Decision Support Tool – Product Description 236
IXICO Plc Pipeline Products & Ongoing Clinical Trials Overview 237
Assessa – Vascular Disease Burden – Product Status 237
Assessa – Vascular Disease Burden – Product Description 237
Johns Hopkins Kimmel Cancer Center Pipeline Products & Ongoing Clinical Trials Overview 239
CompCyst – Product Status 239
CompCyst – Product Description 239
Jozef Stefan Institute Pipeline Products & Ongoing Clinical Trials Overview 240
HeartMan System – Product Status 240
HeartMan System – Product Description 240
King’s College London Pipeline Products & Ongoing Clinical Trials Overview 241
Automatic Brain Abnormality Detection Tool – Product Status 241
Automatic Brain Abnormality Detection Tool – Product Description 241
Koios Medical Pipeline Products & Ongoing Clinical Trials Overview 242
Koios DS – Lung – Product Status 242
Koios DS – Lung – Product Description 242
Thyroid DS – Product Status 243
Thyroid DS – Product Description 243
Koninklijke Philips NV Pipeline Products & Ongoing Clinical Trials Overview 244
AI Based Smart Cathlab Assistance – Product Status 244
AI Based Smart Cathlab Assistance – Product Description 244
Prostate Cancer Care Application – Product Status 245
Prostate Cancer Care Application – Product Description 245
Kyung Hee University Pipeline Products & Ongoing Clinical Trials Overview 246
AI Decision-Making Tool – Liposuction – Product Status 246
AI Decision-Making Tool – Liposuction – Product Description 246
Logical Images Inc Pipeline Products & Ongoing Clinical Trials Overview 247
Visual Dx System – Ultrasound Imaging – Product Status 247
Visual Dx System – Ultrasound Imaging – Product Description 247
Lucence Diagnostics Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 248
Liver3D – Product Status 248
Liver3D – Product Description 248
Lucile Packard Children’s Hospital Stanford Pipeline Products & Ongoing Clinical Trials Overview 249
DL Tool – Hydrocephalus – Product Status 249
DL Tool – Hydrocephalus – Product Description 249
Macau University of Science and Technology Pipeline Products & Ongoing Clinical Trials Overview 250
IRENE – Product Status 250
IRENE – Product Description 250
Macuject Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 251
AI-Based Clinical Decision Support Software – Product Status 251
AI-Based Clinical Decision Support Software – Product Description 251
Manipal Academy of Higher Education Pipeline Products & Ongoing Clinical Trials Overview 252
Decision Support System – Blood Smear Analysis – Product Status 252
Decision Support System – Blood Smear Analysis – Product Description 252
Massachusetts General Hospital Pipeline Products & Ongoing Clinical Trials Overview 253
CXR-CVD Risk Tool – Product Status 253
CXR-CVD Risk Tool – Product Description 253
VIGORIS Clinical Decision Support System – Product Status 253
VIGORIS Clinical Decision Support System – Product Description 254
Massachusetts General Hospital – Ongoing Clinical Trials Overview 255
VIGORIS Clinical Decision Support System – Live User Acceptance Testing of a Decision Support System to Optimize Blood Pressure Management During Critical Care 256
Max Planck Institute for Biological Cybernetics Pipeline Products & Ongoing Clinical Trials Overview
![]()