Contrast Agents – Pipeline Products by Stage of Development 36
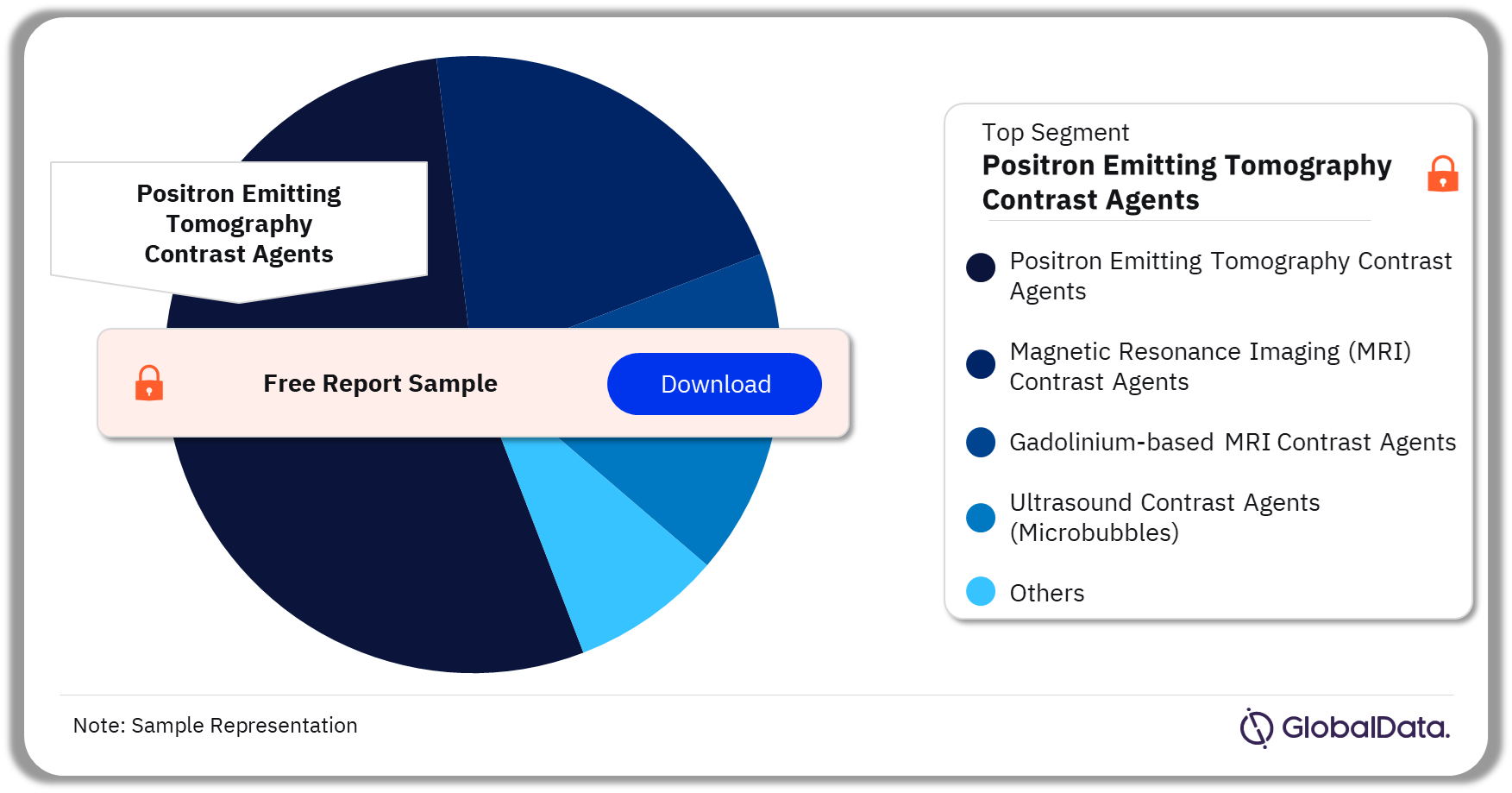
Contrast Agents – Pipeline Products by Segment 37
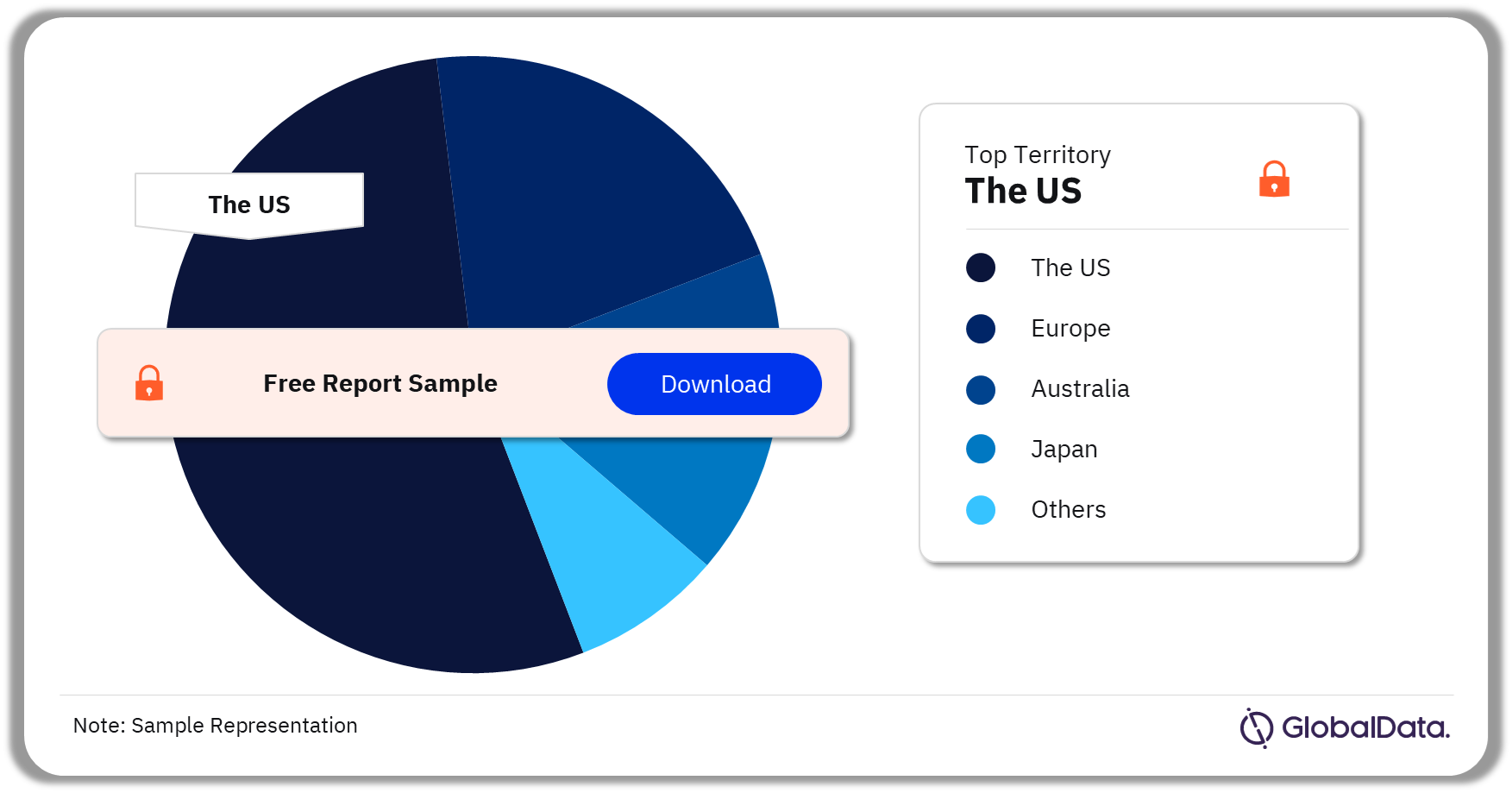
Contrast Agents – Pipeline Products by Territory 39
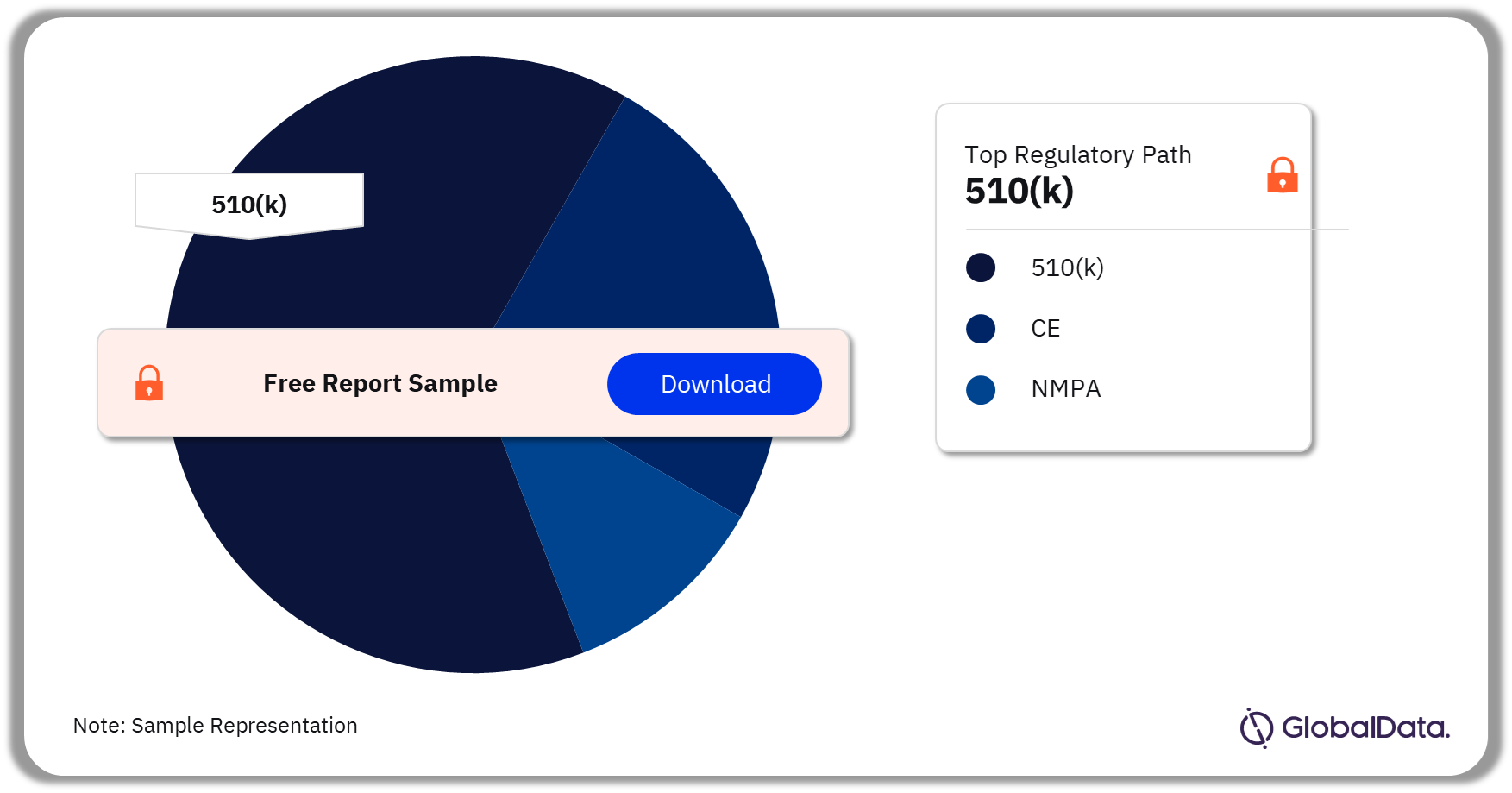
Contrast Agents – Pipeline Products by Regulatory Path 40
Contrast Agents – Pipeline Products by Estimated Approval Date 41
Contrast Agents – Ongoing Clinical Trials 42
Contrast Agents Companies – Pipeline Products by Stage of Development 43
Contrast Agents – Pipeline Products by Stage of Development 51
3B Pharmaceuticals GmbH Pipeline Products & Ongoing Clinical Trials Overview 59
3B 202 – Product Status 59
3B 202 – Product Description 59
3B 222 – Product Status 60
3B 222 – Product Description 60
3B 302 – Product Status 60
3B 302 – Product Description 60
3B 402 – Product Status 61
3B 402 – Product Description 61
3B 502 – Product Status 61
3B 502 – Product Description 62
NN – Product Status 62
NN – Product Description 62
5M Biomed LLC Pipeline Products & Ongoing Clinical Trials Overview 63
Molecular Imaging Probe – Product Status 63
Molecular Imaging Probe – Product Description 63
Surface Functionalized Magnetic Iron Oxide Nanorods – Product Status 64
Surface Functionalized Magnetic Iron Oxide Nanorods – Product Description 64
Water Soluble Magnetic Iron Oxide Nanocubes – Product Status 64
Water Soluble Magnetic Iron Oxide Nanocubes – Product Description 64
Abscint nv Pipeline Products & Ongoing Clinical Trials Overview 65
ABSCINT-206 – Product Status 65
ABSCINT-206 – Product Description 65
ABSCINT-HER2 – Product Status 66
ABSCINT-HER2 – Product Description 66
Abscint nv – Ongoing Clinical Trials Overview 67
ABSCINT-HER2 – A Phase II Clinical Study to Evaluate the ABSCINT-HER2 Imaging Developed for Diagnosis of Single-Domain Antibodies Disease 68
AC Immune SA Pipeline Products & Ongoing Clinical Trials Overview 69
ACI-12589 Imaging Agent – Product Status 69
ACI-12589 Imaging Agent – Product Description 69
TDP-43-PET Tracer – Product Status 70
TDP-43-PET Tracer – Product Description 70
AC Immune SA – Ongoing Clinical Trials Overview 71
ACI-12589 Imaging Agent – A First-in-human Study of ACI-12589 Imaging Agent for Diagnosis of Parkinson’s Disease 72
AccuNovo Biotechnologies Inc Pipeline Products & Ongoing Clinical Trials Overview 73
64Cu Labeled NTSR1-Targeting PET Agent – Product Status 73
64Cu Labeled NTSR1-Targeting PET Agent – Product Description 73
AdAlta Ltd Pipeline Products & Ongoing Clinical Trials Overview 74
Granzyme B PET Imaging Agent – Product Status 74
Granzyme B PET Imaging Agent – Product Description 74
Adamas Nanotechnologies Inc Pipeline Products & Ongoing Clinical Trials Overview 75
Photo-Hyperpolarizable Nanodiamond-Based 13C Contrast Agent – Product Status 75
Photo-Hyperpolarizable Nanodiamond-Based 13C Contrast Agent – Product Description 75
Adiposs SA Pipeline Products & Ongoing Clinical Trials Overview 76
ImageBAT – Product Status 76
ImageBAT – Product Description 76
Aestas Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 77
AP-251 – Product Status 77
AP-251 – Product Description 77
Affibody AB Pipeline Products & Ongoing Clinical Trials Overview 78
GE-226 – Product Status 78
GE-226 – Product Description 78
Akrotome Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 79
FIRE Probe – Breast Cancer – Product Status 79
FIRE Probe – Breast Cancer – Product Description 79
Alume Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 80
ALM-488 – Product Status 80
ALM-488 – Product Description 80
Alume Biosciences Inc – Ongoing Clinical Trials Overview 81
ALM-488 – ALM-488 for Intra-Operative Visualization of Nerves in Head and Neck Surgery 82
Alzeca Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 83
ADx Nanoparticle – Product Status 83
ADx Nanoparticle – Product Description 83
Alzeca Biosciences Inc – Ongoing Clinical Trials Overview 84
ADx Nanoparticle – A Phase 1, Controlled, Open-label, Single Dose, Dose-escalation, Clinical Proof-of-concept Study of MRI Enhanced With ADx-001 (DSPE-DOTA-Gd Liposomal Injection) in Patients with Brain Amyloid Deposits as Demonstrated by Amyloid PET 85
Amydis Inc Pipeline Products & Ongoing Clinical Trials Overview 86
AMDX-201 – Product Status 86
AMDX-201 – Product Description 86
AMDX-2011P – Product Status 87
AMDX-2011P – Product Description 87
Diagnostic Probe – Creutzfeldt-Jakob Disease – Product Status 87
Diagnostic Probe – Creutzfeldt-Jakob Disease – Product Description 88
Fluorescent Retinal Contrast Agent – Parkinson’s Disease – Product Status 88
Fluorescent Retinal Contrast Agent – Parkinson’s Disease – Product Description 88
Fluorescent Retinal Tracer – Product Status 88
Fluorescent Retinal Tracer – Product Description 89
TDP-43 Retinal Tracer – Product Status 89
TDP-43 Retinal Tracer – Product Description 89
Amydis Inc – Ongoing Clinical Trials Overview 90
AMDX-2011P – Open Label, Blinded Endpoint Assessment Study of AMDX-2011P as a Retinal Tracer in Subjects with Cerebral Amyloid Angiopathy (CAA) 91
Fluorescent Retinal Tracer – A Phase I/IIa First-in-human Clinical Study of Retinal Tracer in the Diagnosis and Management of Patients with Amyotrophic Lateral Sclerosis 92
Fluorescent Retinal Contrast Agent – Parkinson’s Disease – Prospective Randomized Open, Blinded Endpoint (PROBE) Study of AMDX-2011P as a Retinal Tracer in Subjects with Neurodegenerative Diseases Associated with Amyloidogenic Proteinopathy 93
Antaros Medical AB Pipeline Products & Ongoing Clinical Trials Overview 94
Gadoxetate DCE-MR Imaging Agent – Liver Toxicity – Product Status 94
Gadoxetate DCE-MR Imaging Agent – Liver Toxicity – Product Description 94
APRINOIA Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 95
APN-1607 – Product Status 95
APN-1607 – Product Description 95
APN-1701 – Product Status 96
APN-1701 – Product Description 96
aSyn PET Tracer – Product Status 96
aSyn PET Tracer – Product Description 97
APRINOIA Therapeutics Inc – Ongoing Clinical Trials Overview 98
APN-1607 – A Phase 3, Multicenter Study of [18F]APN-1607 Positron Emission Tomography in Subjects with Alzheimer’s Disease Compared to Healthy Subjects 99
APN-1607 – A Phase II, Multicenter Study of [18F]APN-1607 Positron Emission Tomography in Subjects with Alzheimer’s Disease Compared to Healthy Subjects 99
APN-1607 – Director of Nuclear Medicine Department 99
APN-1607 – Evaluation of [18F]APN-1607 as a PET Biomarker for Longitudinal Change in Tau Pathology in Participants with Progressive Supranuclear Palsy 100
Ascelia Pharma AB Pipeline Products & Ongoing Clinical Trials Overview 101
Orviglance – Product Status 101
Orviglance – Product Description 101
Second Generation Mangoral – Product Status 102
Second Generation Mangoral – Product Description 102
Ascelia Pharma AB – Ongoing Clinical Trials Overview 103
Orviglance – Study Evaluate the Influence of Hepatic Impairment on the Safety, Pharmacokinetics and Pharmacodynamics of Mangoral 104
Astellas Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 105
ASP5354 – Product Status 105
ASP5354 – Product Description 105
Augurix SA Pipeline Products & Ongoing Clinical Trials Overview 106
AUG-BIO 2 – Product Status 106
AUG-BIO 2 – Product Description 106
AUG-BIO 3 – Product Status 107
AUG-BIO 3 – Product Description 107
Auxano Biomedical, LLC. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 108
Diagnostic Agents – Wounds – Product Status 108
Diagnostic Agents – Wounds – Product Description 108
Avelas Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 109
AVB-N-311 – Product Status 109
AVB-N-311 – Product Description 109
AVB-N-312 – Product Status 110
AVB-N-312 – Product Description 110
B-Aegis Life Sciences & Research Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
OncoSense – Product Status 111
OncoSense – Product Description 111
Bayer AG Pipeline Products & Ongoing Clinical Trials Overview 112
Gadoquatrane Contrast Agent – Product Status 112
Gadoquatrane Contrast Agent – Product Description 112
Next Generation Liver MRI Contrast Agent – Product Status 113
Next Generation Liver MRI Contrast Agent – Product Description 113
BBS NanoTechnology Ltd Pipeline Products & Ongoing Clinical Trials Overview 114
FCA1 – Gd Containing Paramagnetic MR T1 Contrast Agent – Product Status 114
FCA1 – Gd Containing Paramagnetic MR T1 Contrast Agent – Product Description 114
Beckman Research Institute Of The City Of Hope Pipeline Products & Ongoing Clinical Trials Overview 115
Calreticulin ImmunoPET Probe – Product Status 115
Calreticulin ImmunoPET Probe – Product Description 115
Biogen Inc Pipeline Products & Ongoing Clinical Trials Overview 116
Tau Imaging Agent – Product Status 116
Tau Imaging Agent – Product Description 116
Blaze Bioscience Inc Pipeline Products & Ongoing Clinical Trials Overview 117
BLZ-100 – Product Status 117
BLZ-100 – Product Description 117
Bracco Imaging SpA Pipeline Products & Ongoing Clinical Trials Overview 118
Iomeron Contrast Agent – Product Status 118
Iomeron Contrast Agent – Product Description 118
Brown University Pipeline Products & Ongoing Clinical Trials Overview 119
MR Imaging Probe – Cancer – Product Status 119
MR Imaging Probe – Cancer – Product Description 119
Nanobubble Contrast Agent – Product Status 120
Nanobubble Contrast Agent – Product Description 120
Case Western Reserve University Pipeline Products & Ongoing Clinical Trials Overview 121
Contrast Agent – Prostate Cancer – Product Status 121
Contrast Agent – Prostate Cancer – Product Description 121
PET Imaging Agent – Product Status 122
PET Imaging Agent – Product Description 122
ZD2-Gd3N@C80 – Product Status 122
ZD2-Gd3N@C80 – Product Description 122
Cavatar LLC Pipeline Products & Ongoing Clinical Trials Overview 123
99mTc Based Contrast Agent – Product Status 123
99mTc Based Contrast Agent – Product Description 123
Cell2in Pipeline Products & Ongoing Clinical Trials Overview 124
Nuclear GSH Tracer – Product Status 124
Nuclear GSH Tracer – Product Description 124
Cellectar Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 125
CLR 1502 – Product Status 125
CLR 1502 – Product Description 125
Centre National de la Recherche Scientifique Pipeline Products & Ongoing Clinical Trials Overview 126
Integrin Receptor Targeting Fluorescent Imaging Agent – Tumor Diagnosis – Product Status 126
Integrin Receptor Targeting Fluorescent Imaging Agent – Tumor Diagnosis – Product Description 126
Centurion BioPharma Corp Pipeline Products & Ongoing Clinical Trials Overview 127
ACDx – Product Status 127
ACDx – Product Description 127
Cerveau Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 128
NAV4694 – Product Status 128
NAV4694 – Product Description 128
Cerveau Technologies Inc – Ongoing Clinical Trials Overview 129
NAV4694 – Alzheimer’s Disease Neuroimaging Initiative 4 (ADNI4) 130
Chinese Academy of Sciences Pipeline Products & Ongoing Clinical Trials Overview 131
SNAB Probe – Product Status 131
SNAB Probe – Product Description 131
Clarity Pharmaceuticals Ltd Pipeline Products & Ongoing Clinical Trials Overview 132
PET Imaging Agent – Amyotrophic Lateral Sclerosis/Brain Perfusion – Product Status 132
PET Imaging Agent – Amyotrophic Lateral Sclerosis/Brain Perfusion – Product Description 132
PET Imaging Agent – Fibrosis Panel – Product Status 133
PET Imaging Agent – Fibrosis Panel – Product Description 133
ClearNano Inc Pipeline Products & Ongoing Clinical Trials Overview 134
ClearGold – Product Status 134
ClearGold – Product Description 134
ClearICG (Indocyanine Green) – Product Status 134
ClearICG (Indocyanine Green) – Product Description 135
Collagen Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 136
[68Ga]CBP8 – Product Status 136
[68Ga]CBP8 – Product Description 136
68Ga-CM500 – Product Status 136
68Ga-CM500 – Product Description 137
Cosmo Pharmaceuticals NV Pipeline Products & Ongoing Clinical Trials Overview 138
Lumeblue – Product Status 138
Lumeblue – Product Description 138
Curadel LLC Pipeline Products & Ongoing Clinical Trials Overview 139
cRWD-ZW800-1 – Product Status 139
cRWD-ZW800-1 – Product Description 139
PanLN Forte-800 – Product Status 140
PanLN Forte-800 – Product Description 140
SLN-800 – Product Status 140
SLN-800 – Product Description 140
ZW800-1 – Product Status 141
ZW800-1 – Product Description 141
ZW800-1 – Prostate Cancer – Product Status 141
ZW800-1 – Prostate Cancer – Product Description 142
CytoSite BioPharma Inc Pipeline Products & Ongoing Clinical Trials Overview 143
Diagnostic Imaging Agent – Product Status 143
Diagnostic Imaging Agent – Product Description 143
Daewoong Pharmaceutical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 144
Iodixanol Contrast Medium – Product Status 144
Iodixanol Contrast Medium – Product Description 144
Ioversol Contrast Medium – Product Status 145
Ioversol Contrast Medium – Product Description 145
Daimroc Imaging Pipeline Products & Ongoing Clinical Trials Overview 146
Silver Nanoparticle Contrast Agent – Product Status 146
Silver Nanoparticle Contrast Agent – Product Description 146
Diaprost AB Pipeline Products & Ongoing Clinical Trials Overview 147
Mab-PET-Ligand – Hu11b6 – Product Status 147
Mab-PET-Ligand – Hu11b6 – Product Description 147
Drexel University Pipeline Products & Ongoing Clinical Trials Overview 148
Ultrasound Contrast Agent – Product Status 148
Ultrasound Contrast Agent – Product Description 148
Duke University Pipeline Products & Ongoing Clinical Trials Overview 149
18F – Radiolabeled Imaging Agent – Product Status 149
18F – Radiolabeled Imaging Agent – Product Description 149
Edinburgh Molecular Imaging Ltd Pipeline Products & Ongoing Clinical Trials Overview 150
EMI-137 – Product Status 150
EMI-137 – Product Description 151
EMI-200 – Product Status 151
EMI-200 – Product Description 151
EMI-300/350 – Product Status 152
EMI-300/350 – Product Description 152
EMI-400 – Product Status 152
EMI-400 – Product Description 152
EMI-500 – Product Status 153
EMI-500 – Product Description 153
EMI-600 – Product Status 153
EMI-600 – Product Description 153
EMP-100 – Product Status 154
EMP-100 – Product Description 154
EMT-100 – Product Status 154
EMT-100 – Product Description 154
EMT-101 – Product Status 155
EMT-101 – Product Description 155
Emory University Pipeline Products & Ongoing Clinical Trials Overview 156
Imaging Agent – Myocarditis – Product Status 156
Imaging Agent – Myocarditis – Product Description 156
PET Tracer – Parkinson’s Disease – Product Status 157
PET Tracer – Parkinson’s Disease – Product Description 157
Enigma Biomedical Group Inc Pipeline Products & Ongoing Clinical Trials Overview 158
[11C]MK-6884 – Product Status 158
[11C]MK-6884 – Product Description 158
CBD2115 – Product Status 158
CBD2115 – Product Description 159
EOS Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 160
Eos-002 – Product Status 160
Eos-002 – Product Description 160
Eos-004 – Product Status 161
Eos-004 – Product Description 161
Expesicor Inc Pipeline Products & Ongoing Clinical Trials Overview 162
EXP-1801 – Product Status 162
EXP-1801 – Product Description 162
Ferric Contrast Inc Pipeline Products & Ongoing Clinical Trials Overview 163
K720 – Kidney Tumors – Product Status 163
K720 – Kidney Tumors – Product Description 163
L410 – Liver Tumor – Product Status 163
L410 – Liver Tumor – Product Description 164
MP89 – Multipurpose – Product Status 164
MP89 – Multipurpose – Product Description 164
GE HealthCare Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 165
Manganese-Based Macrocyclic Magnetic Resonance Imaging (MRI) Contrast Agent – Product Status 165
Manganese-Based Macrocyclic Magnetic Resonance Imaging (MRI) Contrast Agent – Product Description 165
Non-Lyophilised Sonazoid – Product Status 166
Non-Lyophilised Sonazoid – Product Description 166
GE HealthCare Technologies Inc – Ongoing Clinical Trials Overview 167
Manganese-Based Macrocyclic Magnetic Resonance Imaging (MRI) Contrast Agent – A Phase I Study to Assess the Safety Profile of Injectable Manganese Contrast Agent 168
Genentech USA Inc Pipeline Products & Ongoing Clinical Trials Overview 169
[18F]GTP1 – Product Status 169
[18F]GTP1 – Product Description 169
Georgia State University Pipeline Products & Ongoing Clinical Trials Overview 170
Protein Contrast Agent – Product Status 170
Protein Contrast Agent – Product Description 170
H. Lee Moffitt Cancer Center & Research Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 171
Fluorescent Probe – Pancreatic Cancer – Product Status 171
Fluorescent Probe – Pancreatic Cancer – Product Description 171
Hana Pharm Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 172
HNP-2006 – Product Status 172
HNP-2006 – Product Description 172
Harvard University Pipeline Products & Ongoing Clinical Trials Overview 173
Hyperpolarized MRI Contrast Agent – Product Status 173
Hyperpolarized MRI Contrast Agent – Product Description 173
SPECT Imaging Agent – Neurological Diseases – Product Status 174
SPECT Imaging Agent – Neurological Diseases – Product Description 174
Hebrew University of Jerusalem Pipeline Products & Ongoing Clinical Trials Overview 175
CT Molecular Imaging Probe – Product Status 175
CT Molecular Imaging Probe – Product Description 175
Helmholtz Center Dresden Rossendorf Pipeline Products & Ongoing Clinical Trials Overview 176
Functionalized Silicon Nanoparticles – Product Status 176
Functionalized Silicon Nanoparticles – Product Description 176
Helmholtz Center Munich German Research Center for Health and Environment GmbH Pipeline Products & Ongoing Clinical Trials Overview 177
Black Nanoparticles – Product Status 177
Black Nanoparticles – Product Description 177
Hong Kong Baptist University Pipeline Products & Ongoing Clinical Trials Overview 178
Iridium(III) – Based Probe – Product Status 178
Iridium(III) – Based Probe – Product Description 178
Hong Kong Polytechnic University Pipeline Products & Ongoing Clinical Trials Overview 179
Imaging-Guided Nanoparticle – Product Status 179
Imaging-Guided Nanoparticle – Product Description 179
Houston Methodist Research Institute Pipeline Products & Ongoing Clinical Trials Overview 180
pH-Sensitive Dye – Product Status 180
pH-Sensitive Dye – Product Description 180
Hugo W. Moser Research Institute at Kennedy Krieger Inc Pipeline Products & Ongoing Clinical Trials Overview 181
D-Glucose Contrast Agent – Product Status 181
D-Glucose Contrast Agent – Product Description 181
IC Targets AS Pipeline Products & Ongoing Clinical Trials Overview 182
Mangafodipir – Product Status 182
Mangafodipir – Product Description 182
IC Targets AS – Ongoing Clinical Trials Overview 183
Mangafodipir – Investigation of Blood-brain-barrier Breakdown Using Manganese Magnetic Resonance Imaging in Drug-resistant Epilepsy 184
ImaginAb Inc Pipeline Products & Ongoing Clinical Trials Overview 185
89Zr CD8 ImmunoPET – Product Status 185
89Zr CD8 ImmunoPET – Product Description 185
Antibody-Based Imaging Agent – Ovarian Cancer – Product Status 186
Antibody-Based Imaging Agent – Ovarian Cancer – Product Description 186
CD4 Imaging Agent – Product Status 186
CD4 Imaging Agent – Product Description 186
Macrophage Contrast Agent – Product Status 187
Macrophage Contrast Agent – Product Description 187
ImaginAb Inc – Ongoing Clinical Trials Overview 188
89Zr CD8 ImmunoPET – CD8+ Cell Imaging During Neoadjuvant ImmunoTherapy (The C-IT Neo Trial) 189
89Zr CD8 ImmunoPET – Positron Emission Tomography Repeatability Evaluation of Tissue Zirconium 89Zr Crefmirlimab Berdoxam 189
Imagion Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview 190
MagSense HER2+ Breast Cancer Imaging Agent – Product Status 190
MagSense HER2+ Breast Cancer Imaging Agent – Product Description 190
Magsense Prostate Cancer Imaging Agent – Product Status 191
Magsense Prostate Cancer Imaging Agent – Product Description 191
Imagion Biosystems Inc – Ongoing Clinical Trials Overview 192
MagSense HER2+ Breast Cancer Imaging Agent – A Phase I Study Investigating the Safety and Efficacy of the MagSense Human Epidermal Growth Factor Receptor 2 (HER2) Test Reagent Using Magnetic Resonance Imaging and the MagSense Instrument in Subjects with HER2-positive Breast Cancer to Test for Ipsilateral Lymph Node Involvement 193
iMax Diagnostic Imaging Ltd Pipeline Products & Ongoing Clinical Trials Overview 194
Gadopentetate Dimeglumine – Product Status 194
Gadopentetate Dimeglumine – Product Description 194
Macrocyclic MRI Contrast Agent – Product Status 194
Macrocyclic MRI Contrast Agent – Product Description 195
IMRCP Laboratory Pipeline Products & Ongoing Clinical Trials Overview 196
Contrast Agent – Product Status 196
Contrast Agent – Product Description 196
Indi Molecular Inc Pipeline Products & Ongoing Clinical Trials Overview 197
IO Binding Agent – Product Status 197
IO Binding Agent – Product Description 197
Indian Council of Medical Research Pipeline Products & Ongoing Clinical Trials Overview 198
Imaging Agent – Alzheimer’s Disease – Product Status 198
Imaging Agent – Alzheimer’s Disease – Product Description 198
Indian Institute of Technology Delhi Pipeline Products & Ongoing Clinical Trials Overview 199
MRI Imaging Iron Nano-Particles – Product Status 199
MRI Imaging Iron Nano-Particles – Product Description 199
Inflazome Ltd Pipeline Products & Ongoing Clinical Trials Overview 200
NLRP3 – Product Status 200
NLRP3 – Product Description 200
Inherent Targeting Inc Pipeline Products & Ongoing Clinical Trials Overview 201
Near Infrared Nerve-Specific Fluorophore – Product Status 201
Near Infrared Nerve-Specific Fluorophore – Product Description 201
Inlighta Biosciences LLC Pipeline Products & Ongoing Clinical Trials Overview 202
Novel Contrast Agent – Lung Fibrosis – Product Status 202
Novel Contrast Agent – Lung Fibrosis – Product Description 202
ProCA32.Collagen – Product Status 203
ProCA32.Collagen – Product Description 203
ProCA32.collagen+ – Product Status 203
ProCA32.collagen+ – Product Description 203
ProCA32.CXCR4 – Product Status 204
ProCA32.CXCR4 – Product Description 204
ProCA32.EGFR – Product Status 204
ProCA32.EGFR – Product Description 204
ProCA32.GRPR – Product Status 205
ProCA32.GRPR – Product Description 205
ProCA32.HER2 – Product Status 205
ProCA32.HER2 – Product Description 206
ProCA32.Integrin – Product Status 206
ProCA32.Integrin – Product Description 206
ProCA32.PD-L1 – Product Status 207
ProCA32.PD-L1 – Product Description 207
ProCA32.PSMA – Product Status 207
ProCA32.PSMA – Product Description 207
ProCA32.vEGFR – Product Status 208
ProCA32.vEGFR – Product Description 208
InnoMedica Holding AG Pipeline Products & Ongoing Clinical Trials Overview 209
Contrast Agent – Tumors – Product Status 209
Contrast Agent – Tumors – Product Description 209
Innopharmax Inc Pipeline Products & Ongoing Clinical Trials Overview 210
D0051301 – Product Status 210
D0051301 – Product Description 210
D0131502 – Product Status 211
D0131502 – Product Description 211
Intuitive Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview 212
IS-001 Fluorescent Imaging Agent – Product Status 212
IS-001 Fluorescent Imaging Agent – Product Description 212
Intuitive Surgical Inc – Ongoing Clinical Trials Overview 213
IS-001 Fluorescent Imaging Agent – A Phase 2 Multi-Center Safety and Feasibility Study of IS-001 Injection in Patients Undergoing Robotic-Assisted Colorectal Surgery Using the da Vinci® Surgical System With Firefly® Fluorescent Imaging 214
Inventera Pharmaceutical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 215
CT Contrast Agent – Product Status 215
CT Contrast Agent – Product Description 215
INV-001 – Product Status 216
INV-001 – Product Description 216
INV-002 – Product Status 216
INV-002 – Product Description 217
INV-003 – Product Status 217
INV-003 – Product Description 217
Nuclear Medicine Contrast Agent – Product Status 218
Nuclear Medicine Contrast Agent – Product Description 218
Iowa State University Pipeline Products & Ongoing Clinical Trials Overview 219
Gd5Si4 MRI Contrast Agent – Product Status 219
Gd5Si4 MRI Contrast Agent – Product Description 219
Multimodal Contrast Agent – Product Status 220
Multimodal Contrast Agent – Product Description 220
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 221
4th Generation PSA Test – Product Status 221
4th Generation PSA Test – Product Description 221
PET Imaging Agent – Product Status 222
PET Imaging Agent – Product Description 222
PSMA-Targeted PA Imaging Contrast Agent – Prostate Cancer – Product Status 222
PSMA-Targeted PA Imaging Contrast Agent – Prostate Cancer – Product Description 222
J-Pharma Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 223
NKO-028 – PET Diagnostic Probe – Product Status 223
NKO-028 – PET Diagnostic Probe – Product Description 223
NKO-035 – PET Diagnostic Probe – Product Status 224
NKO-035 – PET Diagnostic Probe – Product Description 224
King’s College London Pipeline Products & Ongoing Clinical Trials Overview 225
Magnetic Resonance Contrast Agent – Product Status 225
Magnetic Resonance Contrast Agent – Product Description 225
Knowledgepie Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 226
Gd-Based Contrast Agent – Product Status 226
Gd-Based Contrast Agent – Product Description 226
Lantheus Medical Imaging Inc Pipeline Products & Ongoing Clinical Trials Overview 227
LMI 1174 – Product Status 227
LMI 1174 – Product Description 227
RP782 – Product Status 228
RP782 – Product Description 228
Life Molecular Imaging SA Pipeline Products & Ongoing Clinical Trials Overview 229
18F-PI-2620 – Product Status 229
18F-PI-2620 – Product Description 229
Life Molecular Imaging SA – Ongoing Clinical Trials Overview 230
18F-PI-2620 – A Brain Imaging Research Study Using NeuraCeq and PI-2620 in Aging Individuals of Thailand 231
18F-PI-2620 – Alzheimer’s Disease Neuroimaging Initiative 4 (ADNI4) 231
18F-PI-2620 – An Open Label, Single Center Study to Evaluate the Safety and Test-retest Characteristics of [18F]PI-2620 as PET Radioligand for Imaging Tau Deposition in the Brains of Patients with Progressive Supranuclear Palsy Richardson Syndrome (PSP-RS) Compared to Non-demented Controls (NDC) 231
18F-PI-2620 – An Open-label, Non-randomized, Multi-center Pivotal Phase 3 Study to Evaluate the Efficacy and Safety of PET Imaging With [18F]PI-2620 for the Detection of Tau Deposition When Compared to Post-mortem Histopathology (ADvance) 232
18F-PI-2620 – First in Human Characterization of PI-2620, a next Generation Pet Tracer for Assessing Tau in Alzheimer’s Disease and Other Tauopathies 232
18F-PI-2620 – Health and Aging Brain Study: Health Disparities Tau PET Scan Study 232
18F-PI-2620 – Imaging [18F]PI-2620 and [18F]Florbetaben in Military Service Members With Blast Related Mild Traumatic Brain Injury 233
18F-PI-2620 – Imaging Tau Accumulation in FTLD and Atypical Alzheimer’s Disease Using the PET Ligand PI-2620 233
18F-PI-2620 – Pilot Phase 1 Clinical Trial of PET Scanning in Tau Protein Deposition and Connectome Analysis in Traumatic Encephalopathy Syndrome (TES) Cohort With a Probable Chronic Traumatic Encephalopathy (CTE) Pattern of Neurodegenerative Disease 233
Ligand Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 234
Captisol-Enabled (CE) Iohexol – Product Status 234
Captisol-Enabled (CE) Iohexol – Product Description 234
Lightpoint Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 235
HARLI – Product Status 235
HARLI – Product Description 235
Lipella Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 236
LP – 20 – Product Status 236
LP – 20 – Product Description 236
Lipella Pharmaceuticals Inc – Ongoing Clinical Trials Overview 237
LP – 20 – Human Clinical Study of a Novel Bladder MRI Contrast Agent 238
Louisiana State University Pipeline Products & Ongoing Clinical Trials Overview 239
CRC Tumor Imaging Agent – Product Status 239
CRC Tumor Imaging Agent – Product Description 239
Lument AB Pipeline Products & Ongoing Clinical Trials Overview 240
Lumentin 44 – Product Status 240
Lumentin 44 – Product Description 240
Macquarie University Pipeline Products & Ongoing Clinical Trials Overview 241
Nanoparticle Imaging Agent – Product Status 241
Nanoparticle Imaging Agent – Product Description 241
Mallinckrodt Institute of Radiology Pipeline Products & Ongoing Clinical Trials Overview 242
PET Imaging Tracer – Product Status 242
PET Imaging Tracer – Product Description 242
Massachusetts General Hospital Pipeline Products & Ongoing Clinical Trials Overview 243
Mn-PyC3A – Product Status 243
Mn-PyC3A – Product Description 243
MRI Contrast Agent – Product Status 244
MRI Contrast Agent – Product Description 244
Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 245
Metal-Free MRI Contrast Agent – Product Status 245
Metal-Free MRI Contrast Agent – Product Description 245
MRI-Based Calcium Sensor – Product Status 246
MRI-Based Calcium Sensor – Product Description 246
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 247
PET Imaging Agent – Na[18F]PF6 – Product Status 247
PET Imaging Agent – Na[18F]PF6 – Product Description 247
MediBeacon Inc Pipeline Products & Ongoing Clinical Trials Overview 248
MB-102 – Product Status 248
MB-102 – Product Description 248
MB-112 – Product Status 249
MB-112 – Product Description 249
MB-202 – Product Status 249
MB-202 – Product Description 249
MB-212 – Product Status 250
MB-212 – Product Description 250
MB-301 – Product Status 250
MB-301 – Product Description 250
MB-404 – Product Status 251
MB-404 – Product Description 251
MediBeacon Inc – Ongoing Clinical Trials Overview 252
MB-102 – A Pilot Study to Assess the Feasibility and Safety of MB-102 in Ocular Angiography as Compared to Fluorescein Sodium 253
MB-102 – A Randomized, Open Label, 2-Period, Cross-over Study to Evaluate the Bioequivalence of the Oversea Manufactured Sample and Domestic Manufactured Sample in Single Intravenous Dose of MB-102 (Relmapirazin) (Part I), and an Efficacy Study to Assess the Performance of the MediBeacon Transdermal GFR Measurement System and Domestic Manufactured Sample of MB-102 (Relmapirazin) for Evaluation of Kidney Function in Chinese Normal and Renal Compromised Subjects (Part II) 253
MB-102 – An Open Label, Multi-Center, Safety and Pharmacokinetic Bridging Study of MB-102 (Relmapirazin) and the Use of the MediBeacon Transdermal GFR Measurement System Using the TGFR Reusable Sensor With Disposable Adhesive Ring in Normal and Renal Compromised Subjects for the Evaluation of Kidney Function 254
Memorial Sloan Kettering Cancer Center Pipeline Products & Ongoing Clinical Trials Overview 255
Zr-89-Transferrin PET Radiotracer – Product Status 255
Zr-89-Transferrin PET Radiotracer – Product Description 255
Merck & Co Inc Pipeline Products & Ongoing Clinical Trials Overview 256
[18F]MK-6240 – Product Status 256
[18F]MK-6240 – Product Description 256
Merck & Co Inc – Ongoing Clinical Trials Overview 257
[18F]MK-6240 – A Safety and Pharmacokinetics Study of [F-18]MK-6240 in Japanese Patients with Mild Cognitive Impairment and Alzheimer’s Disease 258
[18F]MK-6240 – Alzheimer’s Disease Neuroimaging Initiative 4 (ADNI4) 258
[18F]MK-6240 – Longitudinal Multicenter Head-to-Head Harmonization of Tau PET Tracers 258
[18F]MK-6240 – Multicenter Study of Tau Imaging with the Use of [18F]MK-6240 Tracer in Individuals at Risk for and With Autosomal Dominant Alzheimer’s Disease 259
[18F]MK-6240 – The BEACoN Study- Biomarker Exploration in Aging, Cognition and Neurodegeneration 259
[18F]MK-6240 – The Down Syndrome Clinical Trials Network (DS-CTN) Study of Alzheimer’s Disease in Down Syndrome 259
Microbial Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 260
PET Imaging Agent – Product Status 260
PET Imaging Agent – Product Description 260
Microvascular Therapeutics LLC Pipeline Products & Ongoing Clinical Trials Overview 261
MVT-100 – Product Status 261
MVT-100 – Product Description 261
MVT-101 – Product Status 262
MVT-101 – Product Description 262
MVT-102 – Product Status 262
MVT-102 – Product Description 262
MVT-103 – Product Status 263
MVT-103 – Product Description 263
MVT-104 – Product Status 263
MVT-104 – Product Description 263
MVT-105 – Product Status 264
MVT-105 – Product Description 264
Molecular Theranostics LLC Pipeline Products & Ongoing Clinical Trials Overview 265
MRI Contrast Agent – Prostate Cancer (MT218) – Product Status 265
MRI Contrast Agent – Prostate Cancer (MT218) – Product Description 265
MT216 – Product Status 265
MT216 – Product Description 266
MTP219 – Product Status 266
MTP219 – Product Description 266
Monash University Pipeline Products & Ongoing Clinic
![]()