Electrophysiology Ablation Catheters Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Electrophysiology Ablation Catheters Pipeline Market Report Overview
Electrophysiology ablation is applicable for a wide variety of arrhythmias and has an extremely high success rate with low rates of complication and recurrence. The electrophysiology ablation catheters pipeline market research report provides a comprehensive understanding of the pipeline products along with their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Segments | · Advanced Ablation Catheters
· Irrigated tip RF Ablation Catheters · Conventional Radio Frequency Ablation Catheters · Cryoablation Catheters · Laser Ablation Catheters · Standard Ablation Catheters |
| Key Territories | · The US
· Europe · China · Australia · Japan |
| Key Regulatory Paths | · CE
· 510(k) · PMA · NMPA · TGA |
| Leading Companies | · Biosense Webster Inc.
· BioTex Inc. · Ablacon Inc · Acotec Scientific Co Ltd · Adagio Medical Inc. |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Electrophysiology Ablation Catheters Pipeline Market Segmentation by Segments
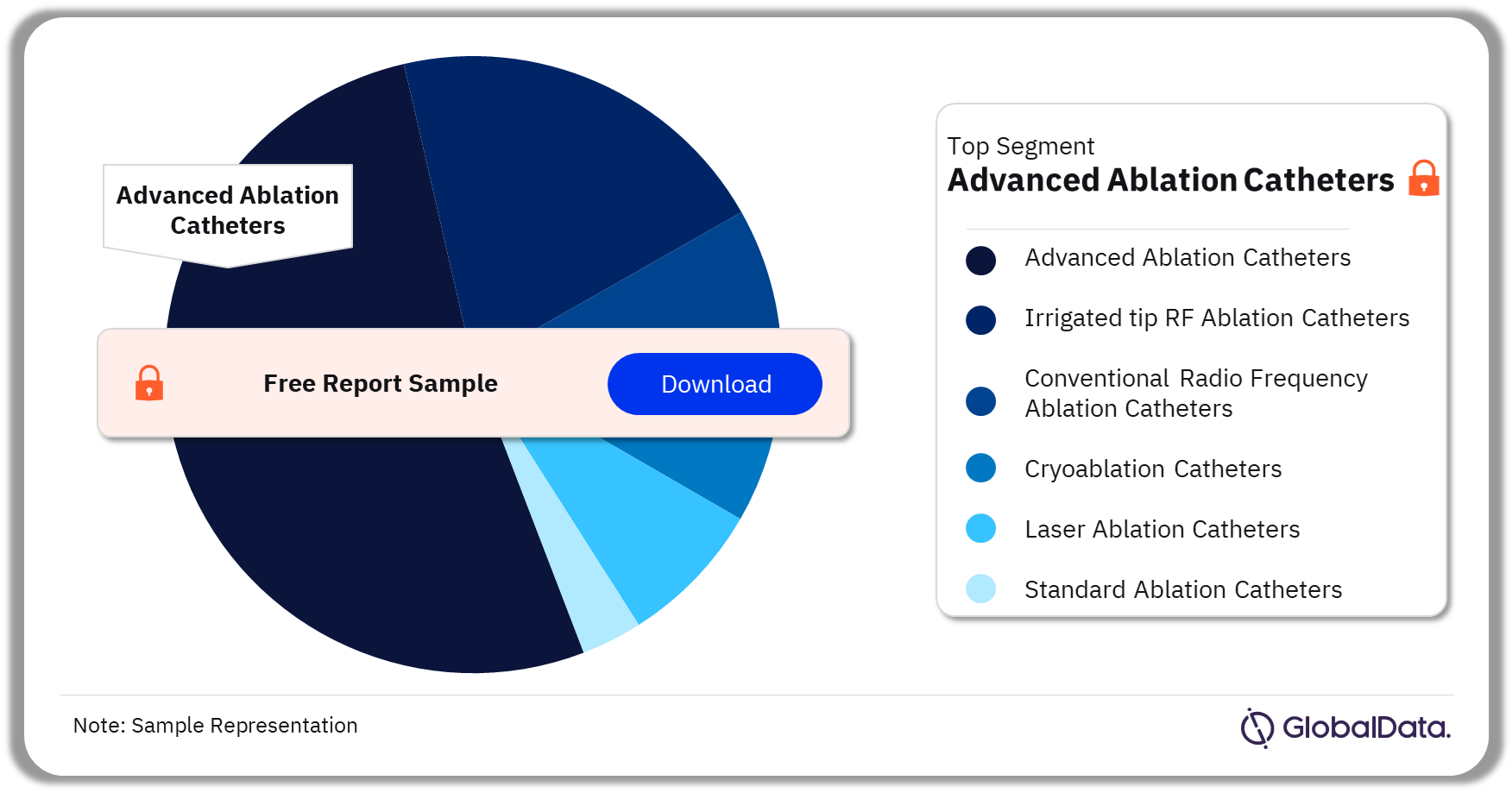
The key segments for the electrophysiology ablation catheters pipeline market are advanced ablation catheters, irrigated tip RF ablation catheters, conventional radiofrequency ablation catheters, cryoablation catheters, laser ablation catheters, and standard ablation catheters. As of March 2024, advanced ablation catheters accounted for the highest share of pipeline products.
Electrophysiology Ablation Catheters Pipeline Market Analysis by Segments, 2024 (%)
Buy the Full Report for More Segment Insights into the Electrophysiology Ablation Catheters Pipeline Market
Electrophysiology Ablation Catheters Pipeline Market Segmentation by Territories
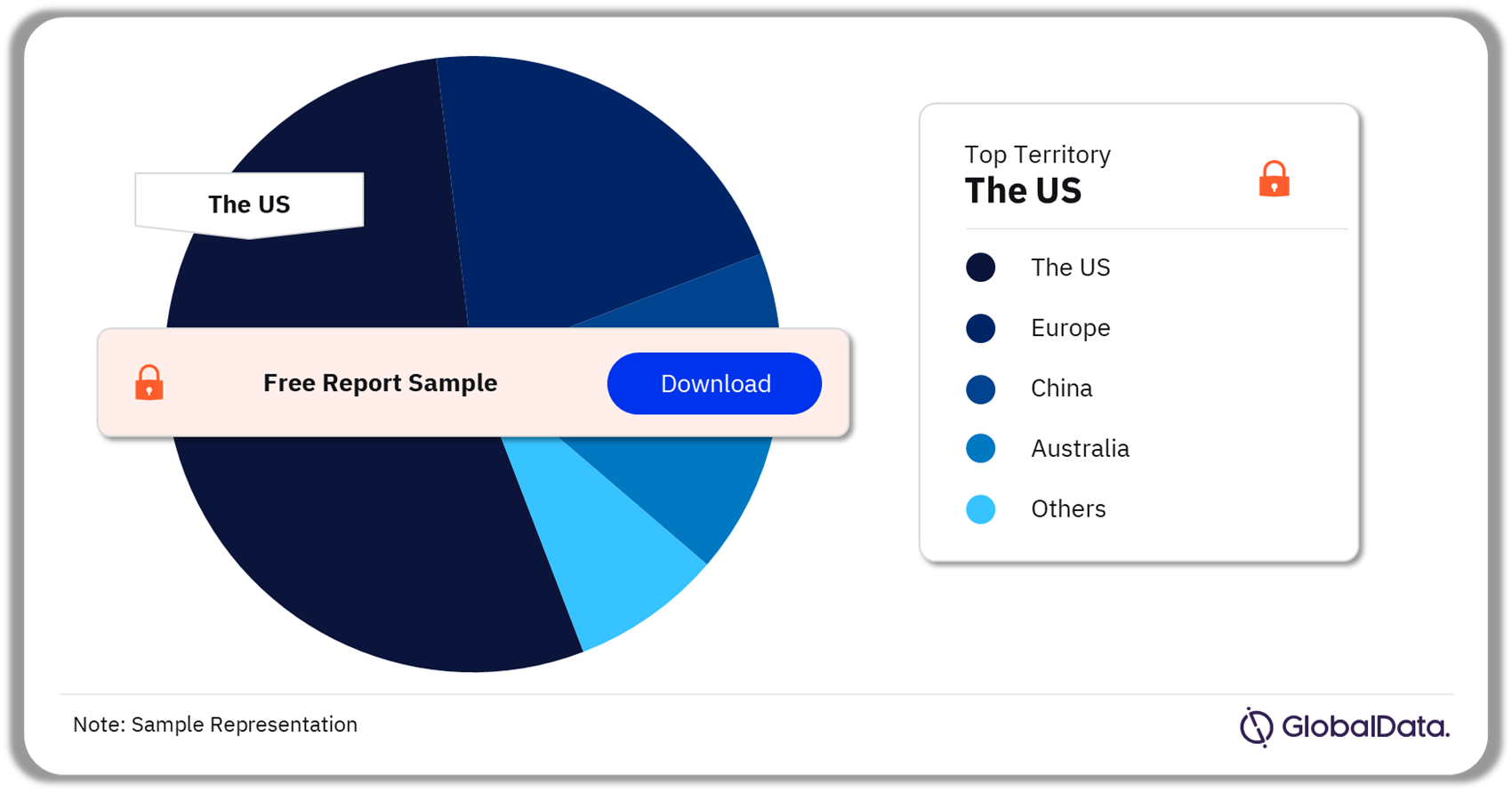
A few of the key territories for the electrophysiology ablation catheters pipeline market are the US, Europe, China, Australia, and Japan. As of March 2024, the US accounted for the highest number of pipeline products.
Electrophysiology Ablation Catheters Pipeline Market Analysis by Territories, 2024 (%)
Buy the Full Report for More Territory Insights into the Electrophysiology Ablation Catheters Pipeline Market
Electrophysiology Ablation Catheters Pipeline Market Segmentation by Regulatory Paths
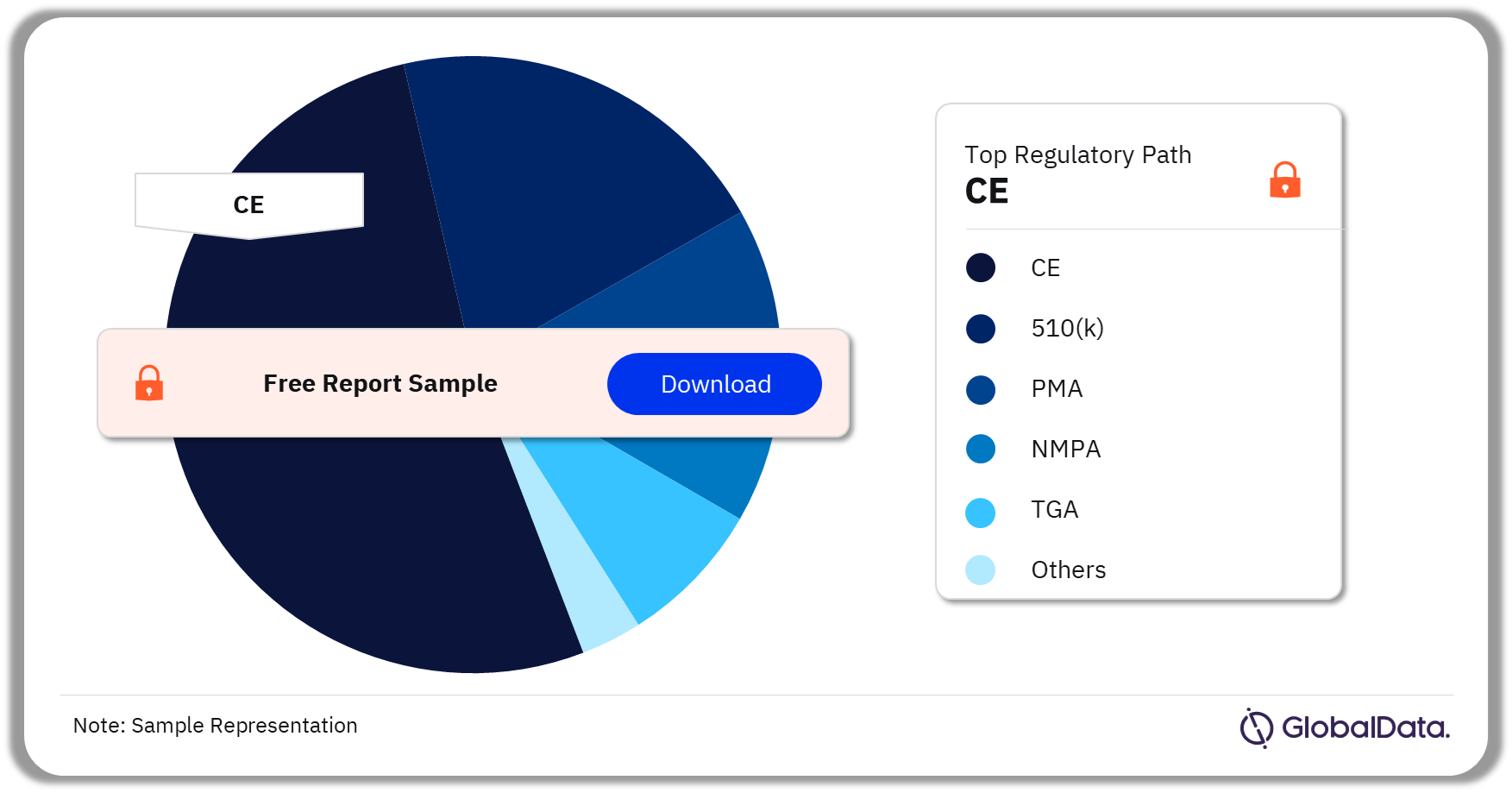
A few of the key regulatory paths in the electrophysiology ablation catheters pipeline products market are CE, 510(k), PMA, NMPA, and TGA. As of March 2024, CE was the most followed pathway for pipeline products in the market.
Electrophysiology Ablation Catheters Pipeline Market Analysis by Regulatory Paths, 2024 (%)
Buy the Full Report for More Regulatory Path Insights into the Electrophysiology Ablation Catheters Pipeline Market
Electrophysiology Ablation Catheters Pipeline Market – Competitive Landscape
A few of the key companies associated with the electrophysiology ablation catheters pipeline market are Biosense Webster Inc., BioTex Inc., Ablacon Inc., Acotec Scientific Co Ltd, and Adagio Medical Inc., among others.
Biosense Webster Inc: Biosense Webster is headquartered in Diamond Bar, California, the US. It is a medical device company that designs, manufactures, and markets cardiac arrhythmias products. Biosense Webster provides onsite training, offsite workshops, online training, EP training, and professional and medical education services. The company offers training and online courses through its education centers in the US, Germany, Belgium, Sweden, and Spain.
BioTex Inc: BioTex is headquartered in Houston, Texas, the US. It operates as a medical device company that designs, develops, manufactures, and distributes electro-mechanical, optical, and software-based devices. The company offers sterile and non-sterile disposable products for surgical, non-surgical, and interventional procedures in the diagnostic, medical device, and life sciences industries.
Electrophysiology Ablation Catheters Pipeline Market Analysis by Companies, 2024
Buy the Full Report for More Company Insights into the Electrophysiology Ablation Catheters Pipeline Market
Segments Covered in the Report
Electrophysiology Ablation Catheters Pipeline Market Segments Outlook
- Advanced Ablation Catheters
- Irrigated tip RF Ablation Catheters
- Conventional Radio Frequency Ablation Catheters
- Cryoablation Catheters
- Laser Ablation Catheters
- Standard Ablation Catheters
Electrophysiology Ablation Catheters Pipeline Market Territories Outlook
- The US
- Europe
- China
- Australia
- Japan
Electrophysiology Ablation Catheters Pipeline Market Regulatory Paths Outlook
- CE
- 510(k)
- PMA
- NMPA
- TGA
Scope
This report provides:
- Extensive coverage of the electrophysiology ablation catheters under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of electrophysiology ablation catheters and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of electrophysiology ablation catheters under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out an in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Acotec Scientific Co Ltd
Adagio Medical Inc.
Apama Medical Inc
APT Medical Inc
Biosense Webster Inc
BioTex Inc
Boston Scientific Corp
CardioFocus Inc
CardioNova Ltd
CathEffects LLC
CathRx Ltd
Cibiem, Inc.
Columbia University
Cordis US Corp
CoreMap Inc
Coridea, LLC
CryoTherapeutics SA
Epicardial Technologies Inc (Inactive)
Ethicon US LLC
Farapulse Inc
FocusStart LLC
Galvanize Therapeutics Inc
Hansen Medical Inc (Inactive)
Hyblate Medical
Imricor Medical Systems Inc
Interventional Imaging, Inc.
Iowa Approach Inc.
Japan Lifeline Co Ltd
Maestro Heart SA
Medlumics SL
MedWaves Inc
MicroPort Scientific Corp
Northwestern University
Novasentis Inc
OrbusNeich
SCR, Inc.
Seattle Children's Hospital
Shanghai HeartCare Medical Technology Co Ltd
Shanghai MicroPort EP MedTech Co Ltd
St. Jude Medical LLC
Stereotaxis Inc
University of Arkansas at Little Rock
University of Michigan
University of Rochester
University of Texas Medical Branch at Galveston
Vanderbilt University
Vimecon GmbH
Vivonics Inc
Voyage Medical (Inactive)
Table of Contents
Table
Figures
Frequently asked questions
-
Which segment had the highest share in the electrophysiology ablation catheters pipeline market as of March 2024?
As of March 2024, advanced ablation catheters accounted for the highest share of electrophysiology ablation catheters pipeline products.
-
Which territory had the highest number of products in the electrophysiology ablation catheters pipeline market as of March 2024?
As of March 2024, the US accounted for the highest number of pipeline products in the electrophysiology ablation catheters market.
-
Which was the most followed regulatory pathway in the electrophysiology ablation catheters pipeline market as of March 2024?
As of March 2024, CE was the most followed pathway for pipeline products in the electrophysiology ablation catheters market.
-
Which are the major companies operating in the electrophysiology ablation catheters pipeline market?
A few of the key companies associated with the electrophysiology ablation catheters pipeline market are Biosense Webster Inc., BioTex Inc., Ablacon Inc., Acotec Scientific Co Ltd, and Adagio Medical Inc., among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Electrophysiology Ablation Catheters reports