Hematological Disorders – Pipeline Products by Stage of Development 21
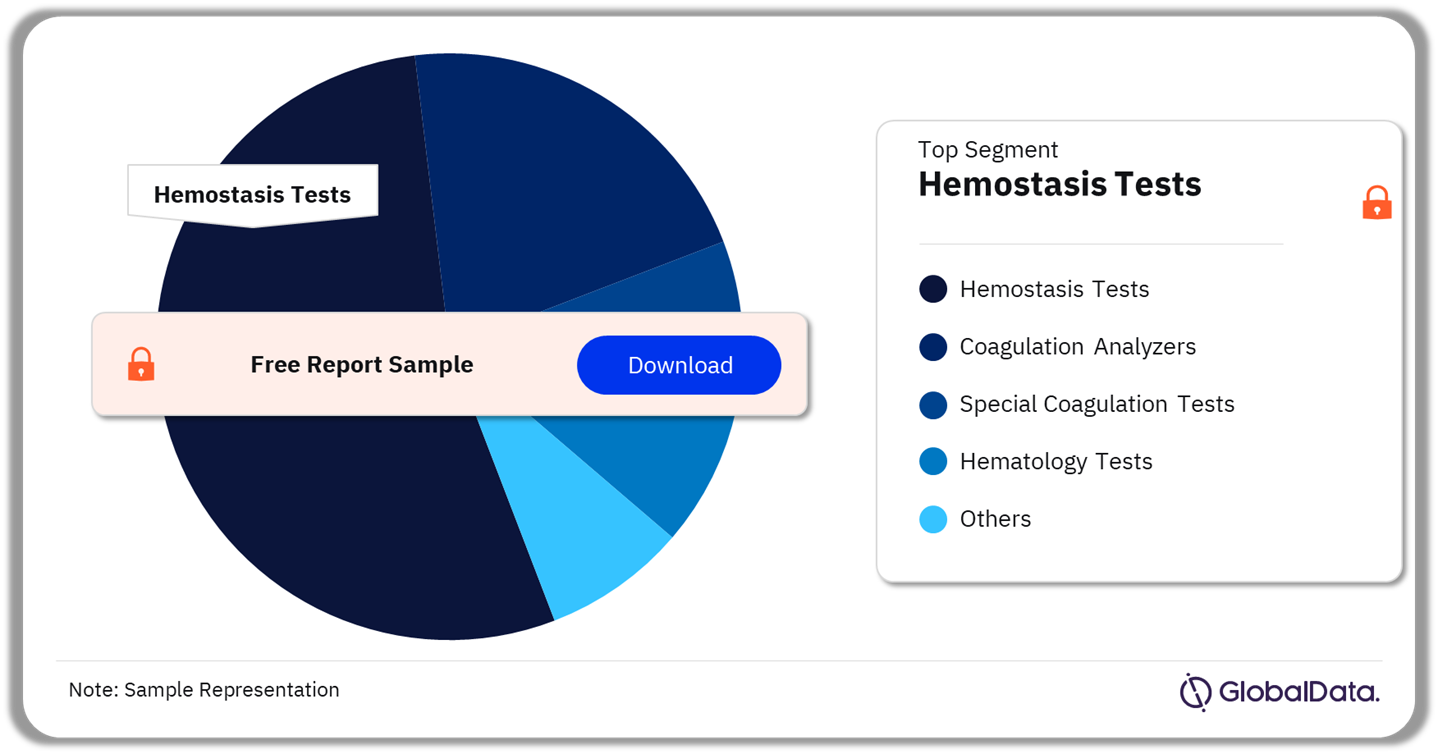
Hematological Disorders – Pipeline Products by Segment 22
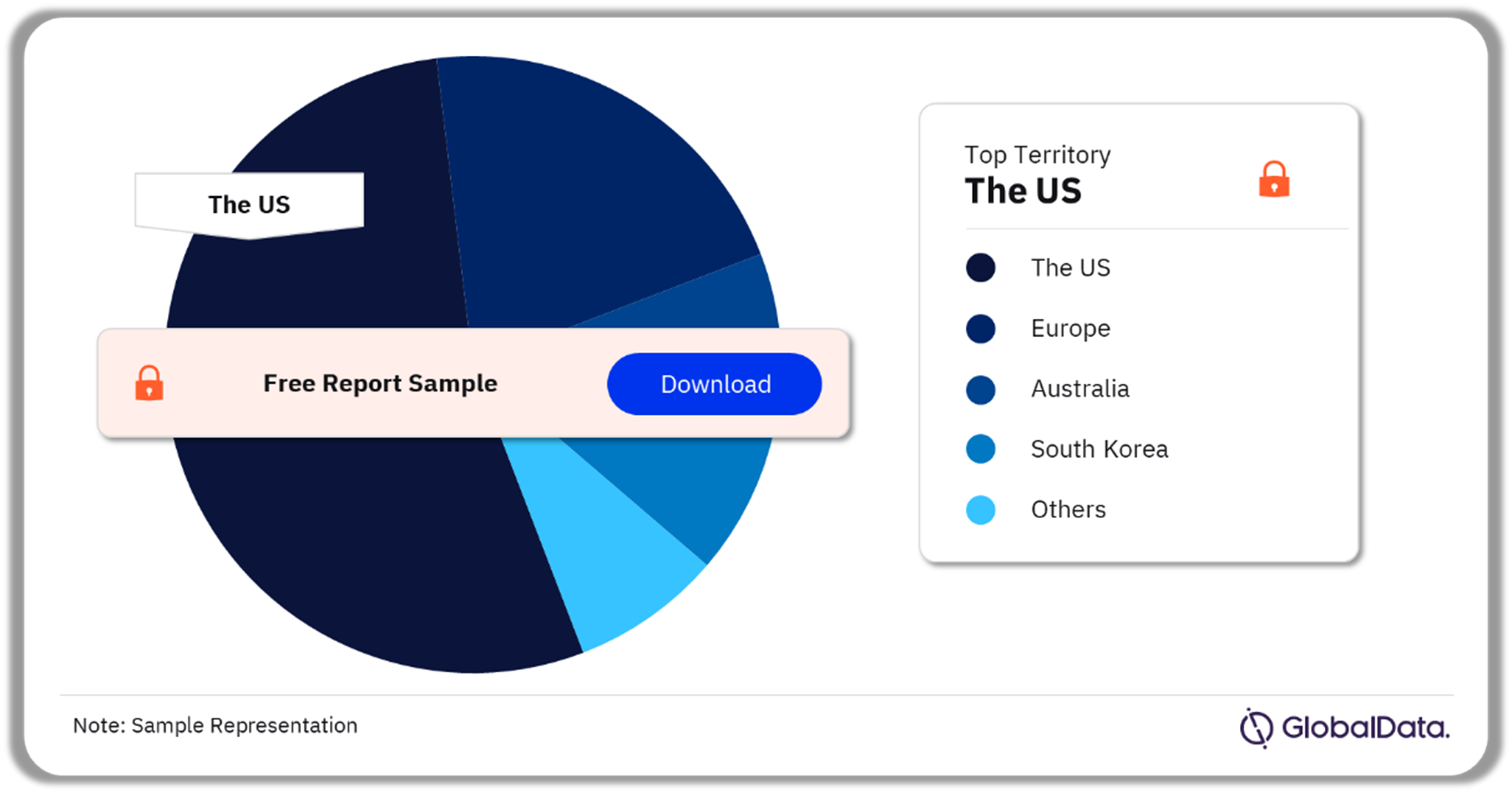
Hematological Disorders – Pipeline Products by Territory 24
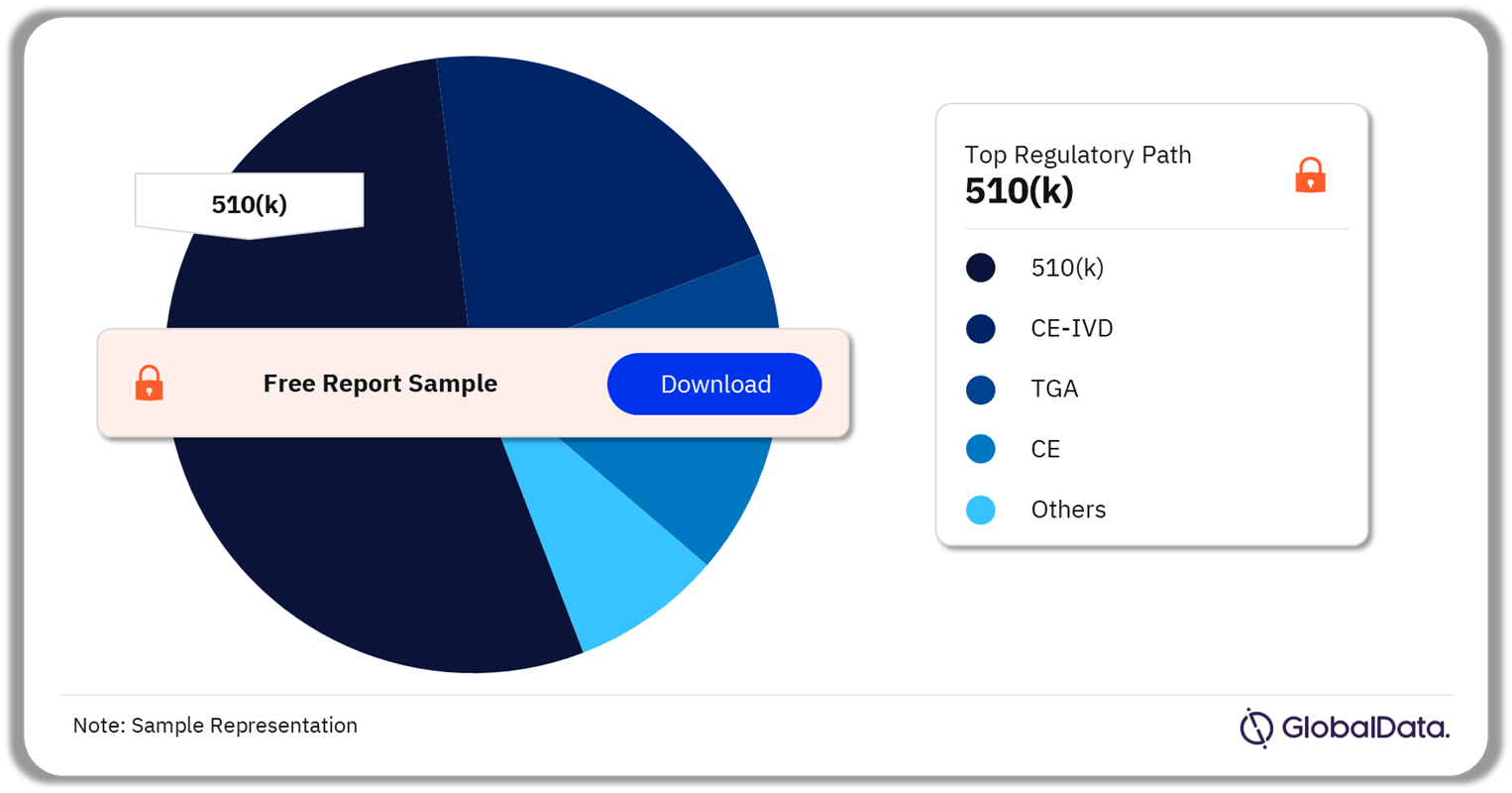
Hematological Disorders – Pipeline Products by Regulatory Path 25
Hematological Disorders – Pipeline Products by Estimated Approval Date 26
Hematological Disorders – Ongoing Clinical Trials 27
Hematological Disorders Companies – Pipeline Products by Stage of Development 28
Hematological Disorders – Pipeline Products by Stage of Development 32
Abionic SA Pipeline Products & Ongoing Clinical Trials Overview 37
Point of Care Diagnostic Test – Basophils – Product Status 37
Point of Care Diagnostic Test – Basophils – Product Description 38
Point of Care Diagnostic Test – Eosinophils – Product Status 38
Point of Care Diagnostic Test – Eosinophils – Product Description 38
Point of Care Diagnostic Test – Erythrocyte – Product Status 39
Point of Care Diagnostic Test – Erythrocyte – Product Description 39
Point of Care Diagnostic Test – Hemoglobin – Product Status 39
Point of Care Diagnostic Test – Hemoglobin – Product Description 40
Point of Care Diagnostic Test – Lymphocytes – Product Status 40
Point of Care Diagnostic Test – Lymphocytes – Product Description 40
Point of Care Diagnostic Test – Neutrophils – Product Status 41
Point of Care Diagnostic Test – Neutrophils – Product Description 41
Point of Care Diagnostic Test – Thrombocytes – Product Status 41
Point of Care Diagnostic Test – Thrombocytes – Product Description 42
Point of Care Diagnostic Test – White Blood Cells – Product Status 42
Point of Care Diagnostic Test – White Blood Cells – Product Description 42
Abram Scientific Inc Pipeline Products & Ongoing Clinical Trials Overview 43
CoagCare System – Product Status 43
CoagCare System – Product Description 43
Alere Inc Pipeline Products & Ongoing Clinical Trials Overview 44
Afinion PT Test – Capillary Blood – Product Status 44
Afinion PT Test – Capillary Blood – Product Description 44
Analyticon Biotechnologies AG Pipeline Products & Ongoing Clinical Trials Overview 45
Coagulyzer 1 – Product Status 45
Coagulyzer 1 – Product Description 46
Coagulyzer 2 – Product Status 46
Coagulyzer 2 – Product Description 47
Coagulyzer 4 – Product Status 47
Coagulyzer 4 – Product Description 48
Aptitude Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 49
POC Test – Trauma Coagulopathy – Product Status 49
POC Test – Trauma Coagulopathy – Product Description 49
Atantares Corp Pipeline Products & Ongoing Clinical Trials Overview 50
Platelet Function Assay – Product Status 50
Platelet Function Assay – Product Description 50
Atomo Diagnostics Ltd Pipeline Products & Ongoing Clinical Trials Overview 51
AtomoRapid – Coagulation Monitoring – Product Status 51
AtomoRapid – Coagulation Monitoring – Product Description 51
Baebies Inc Pipeline Products & Ongoing Clinical Trials Overview 52
Digital Microfluidic System – Trauma Coagulation – Product Status 52
Digital Microfluidic System – Trauma Coagulation – Product Description 52
Beckman Coulter Inc Pipeline Products & Ongoing Clinical Trials Overview 53
SYNCHRON Lxi 725 D-Dimer Assay – Product Status 53
SYNCHRON Lxi 725 D-Dimer Assay – Product Description 53
Beijing Sailing Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 54
Fourth-Generation Thromboelastogrpahy Device – Product Status 54
Fourth-Generation Thromboelastogrpahy Device – Product Description 54
Biochip Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 55
Endothelium-on-a-Chip Assay – Product Status 55
Endothelium-on-a-Chip Assay – Product Description 55
BioMedica USA LLC (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 56
Portable Diagnostic System – Hemostasis – Product Status 56
Portable Diagnostic System – Hemostasis – Product Description 56
BioMedomics Inc Pipeline Products & Ongoing Clinical Trials Overview 57
Fetomaternal Hemorrhage Test – Product Status 57
Fetomaternal Hemorrhage Test – Product Description 57
Hemo SCAN – Product Status 58
Hemo SCAN – Product Description 58
Hemo SCAN S – Product Status 58
Hemo SCAN S – Product Description 59
Rapid Quantitative Heparin-Induced Thrombocytopenia (H.I.T.) Test – Product Status 59
Rapid Quantitative Heparin-Induced Thrombocytopenia (H.I.T.) Test – Product Description 59
Biosurfit, SA Pipeline Products & Ongoing Clinical Trials Overview 60
Spinit Device – Blood Quantitation Anemia Assay – Product Status 60
Spinit Device – Blood Quantitation Anemia Assay – Product Description 60
Spinit Device – Thrombocytopenia Assay – Product Status 61
Spinit Device – Thrombocytopenia Assay – Product Description 61
Braun Biosystems, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 62
Bio6 – Product Status 62
Bio6 – Product Description 62
Carclo Diagnostic Solutions Ltd Pipeline Products & Ongoing Clinical Trials Overview 63
Micropoc-CAT – Haemostasis Assay – Product Status 63
Micropoc-CAT – Haemostasis Assay – Product Description 63
Coramed Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview 64
TEG 6000 Thrombelastograph – Product Status 64
TEG 6000 Thrombelastograph – Product Description 64
Diagnostica Stago Inc Pipeline Products & Ongoing Clinical Trials Overview 65
Calibrated Automated Thrombogram – Thrombin Assay – Product Status 65
Calibrated Automated Thrombogram – Thrombin Assay – Product Description 66
STA Compact Max – NeoPTimal ISI – Product Status 66
STA Compact Max – NeoPTimal ISI – Product Description 66
STA Compact Max – Ristocetin Cofactor Assay – Product Status 67
STA Compact Max – Ristocetin Cofactor Assay – Product Description 67
STA Compact Max – Rivaroxaban Calibrators And Controls For Anti-Xa – Product Status 67
STA Compact Max – Rivaroxaban Calibrators And Controls For Anti-Xa – Product Description 68
STA-R Evolution Expert – Automated Ecarin Chromogenic Assay – Product Status 68
STA-R Evolution Expert – Automated Ecarin Chromogenic Assay – Product Description 68
STA-R Evolution Plus – Automated Ecarin Chromogenic Assay – Product Status 69
STA-R Evolution Plus – Automated Ecarin Chromogenic Assay – Product Description 69
STA-R Evolution Plus – Rivaroxaban and Apixaban Calibrators And Controls For Anti-Xa – Product Status 69
STA-R Evolution Plus – Rivaroxaban and Apixaban Calibrators And Controls For Anti-Xa – Product Description 70
STA-R Max – NeoPTimal ISI – Product Status 70
STA-R Max – NeoPTimal ISI – Product Description 70
STA-R Max – Ristocetin Cofactor Assay – Product Status 71
STA-R Max – Ristocetin Cofactor Assay – Product Description 71
STA-R Max – Rivaroxaban Calibrators And Controls For Anti-Xa – Product Status 71
STA-R Max – Rivaroxaban Calibrators And Controls For Anti-Xa – Product Description 72
DxDiscovery Inc Pipeline Products & Ongoing Clinical Trials Overview 73
Diagnostic Assay – Sickle Cell Disease – Product Status 73
Diagnostic Assay – Sickle Cell Disease – Product Description 73
Dynasil Corporation of America Pipeline Products & Ongoing Clinical Trials Overview 74
Diagnostic Test – Hemophilia – Product Status 74
Diagnostic Test – Hemophilia – Product Description 74
Edan Instruments Inc Pipeline Products & Ongoing Clinical Trials Overview 75
i15 – Coagulation Test – Product Status 75
i15 – Coagulation Test – Product Description 75
EKF Diagnostics Holdings Plc Pipeline Products & Ongoing Clinical Trials Overview 76
Point-Of-Care Test – Coagulation – Product Status 76
Point-Of-Care Test – Coagulation – Product Description 76
ELITechGroup Inc Pipeline Products & Ongoing Clinical Trials Overview 77
Selectra ProM Chemistry System – D-dimer Assay – Product Status 77
Selectra ProM Chemistry System – D-dimer Assay – Product Description 77
Selectra ProS Chemistry System – D-dimer Assay – Product Status 78
Selectra ProS Chemistry System – D-dimer Assay – Product Description 78
Emosis SAS Pipeline Products & Ongoing Clinical Trials Overview 79
ClotSeek – Product Status 79
ClotSeek – Product Description 79
Epimune GmbH Pipeline Products & Ongoing Clinical Trials Overview 80
i.Mune Tbnk Test – Product Status 80
i.Mune Tbnk Test – Product Description 80
Epinex Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 81
Epinex SimplySure PT-INR Test – Product Status 81
Epinex SimplySure PT-INR Test – Product Description 81
ET Healthcare Inc Pipeline Products & Ongoing Clinical Trials Overview 82
Pylon Assay – D-Dimer – Product Status 82
Pylon Assay – D-Dimer – Product Description 82
Flobio LLC Pipeline Products & Ongoing Clinical Trials Overview 83
DOAC Diagnostic Assay – Product Status 83
DOAC Diagnostic Assay – Product Description 83
Fraunhofer Institute for Cell Therapy and Immunology Pipeline Products & Ongoing Clinical Trials Overview 84
Point-Of-Care Assay – Primary And Secondary Hemostasis – Product Status 84
Point-Of-Care Assay – Primary And Secondary Hemostasis – Product Description 84
genedrive plc Pipeline Products & Ongoing Clinical Trials Overview 85
Genedrive – Factor V Leiden – Product Status 85
Genedrive – Factor V Leiden – Product Description 85
Grifols SA Pipeline Products & Ongoing Clinical Trials Overview 86
Hemostasis Reagent Kit – DG-APTT Synth G-Fib L Human – Product Status 86
Hemostasis Reagent Kit – DG-APTT Synth G-Fib L Human – Product Description 86
Hemostasis Reagent Kit – DG-Latex D Dimer – Product Status 87
Hemostasis Reagent Kit – DG-Latex D Dimer – Product Description 87
Hemostasis Reagent Kit – DG-Latex VWF: Gp1b – Product Status 87
Hemostasis Reagent Kit – DG-Latex VWF: Gp1b – Product Description 88
Hemostasis Reagent Kit – DG-TT L Human – Product Status 88
Hemostasis Reagent Kit – DG-TT L Human – Product Description 88
Group K Diagnostics Pipeline Products & Ongoing Clinical Trials Overview 89
KromaHealth Kit – Complete Blood Count – Product Status 89
KromaHealth Kit – Complete Blood Count – Product Description 89
Hansjorg Wyss Institute for Biologically Inspired Engineering Pipeline Products & Ongoing Clinical Trials Overview 90
Hemostasis Monitoring Microfluidic Device – Product Status 90
Hemostasis Monitoring Microfluidic Device – Product Description 90
Helena Laboratories Corp Pipeline Products & Ongoing Clinical Trials Overview 91
Actalyke Mini II – APTT Test – Product Status 92
Actalyke Mini II – APTT Test – Product Description 92
Actalyke Mini II – LMWH Test – Product Status 92
Actalyke Mini II – LMWH Test – Product Description 93
Actalyke Mini II – PT Test – Product Status 93
Actalyke Mini II – PT Test – Product Description 93
Actalyke XL – APTT Test – Product Status 94
Actalyke XL – APTT Test – Product Description 94
Actalyke XL – Heparin Test – Product Status 94
Actalyke XL – Heparin Test – Product Description 95
Actalyke XL – LMWH Test – Product Status 95
Actalyke XL – LMWH Test – Product Description 95
Actalyke XL – PT Test – Product Status 96
Actalyke XL – PT Test – Product Description 96
AggRAM – Chromogenic Test – Product Status 96
AggRAM – Chromogenic Test – Product Description 97
AggRAM – HIT Test – Product Status 97
AggRAM – HIT Test – Product Description 97
AggRAM – Lumi Test – Product Status 97
AggRAM – Lumi Test – Product Description 98
Cascade Abrazo – c-ACT-LR – Product Status 98
Cascade Abrazo – c-ACT-LR – Product Description 98
Cascade Abrazo – Direct Oral Anticoagulants Test – Product Status 99
Cascade Abrazo – Direct Oral Anticoagulants Test – Product Description 99
Cascade Abrazo – Direct Thrombin Inhibitors – Product Status 99
Cascade Abrazo – Direct Thrombin Inhibitors – Product Description 100
Cascade Abrazo – Fibrinogen – Product Status 100
Cascade Abrazo – Fibrinogen – Product Description 100
Cascade Abrazo – Heparin Titration – Product Status 101
Cascade Abrazo – Heparin Titration – Product Description 101
Cascade Abrazo – LMWH – Product Status 101
Cascade Abrazo – LMWH – Product Description 102
Cascade Abrazo – Protamine Titration – Product Status 102
Cascade Abrazo – Protamine Titration – Product Description 102
Cascade Abrazo – PT-C – Product Status 103
Cascade Abrazo – PT-C – Product Description 103
Cascade Abrazo Analyzer – Product Status 103
Cascade Abrazo Analyzer – Product Description 104
Cascade M – DRVVT Test – Product Status 104
Cascade M – DRVVT Test – Product Description 104
Cascade M-4 – DRVVT Test – Product Status 105
Cascade M-4 – DRVVT Test – Product Description 105
hemCheck Sweden AB Pipeline Products & Ongoing Clinical Trials Overview 106
v-Test – Serum Tubes – Product Status 106
v-Test – Serum Tubes – Product Description 106
Hemex Health Inc Pipeline Products & Ongoing Clinical Trials Overview 107
Gazelle Hb Variant Test – Product Status 107
Gazelle Hb Variant Test – Product Description 107
HemoSonics LLC Pipeline Products & Ongoing Clinical Trials Overview 108
HemoSonics Global Hemostasis Analyzer – Intensive Care – Product Status 108
HemoSonics Global Hemostasis Analyzer – Intensive Care – Product Description 108
HemoSonics Global Hemostasis Analyzer – Neurosurgery – Product Status 109
HemoSonics Global Hemostasis Analyzer – Neurosurgery – Product Description 109
HemoSonics Global Hemostasis Analyzer – Orthopedic Surgery – Product Status 109
HemoSonics Global Hemostasis Analyzer – Orthopedic Surgery – Product Description 110
HemoSonics Global Hemostasis Analyzer – Trauma – Product Status 110
HemoSonics Global Hemostasis Analyzer – Trauma – Product Description 110
HemoSonics Global Hemostasis Analyzer HS-GHA – Product Status 111
HemoSonics Global Hemostasis Analyzer HS-GHA – Product Description 111
Immunostep S.L Pipeline Products & Ongoing Clinical Trials Overview 112
Bmplex Hemodilution Test – Product Status 112
Bmplex Hemodilution Test – Product Description 112
Instrumentation Laboratory Co Pipeline Products & Ongoing Clinical Trials Overview 113
HemosIL VWF-Ristocetin Cofactor Activity Assay – Product Status 113
HemosIL VWF-Ristocetin Cofactor Activity Assay – Product Description 113
IntraMed Diagnostics, LLC Pipeline Products & Ongoing Clinical Trials Overview 114
ProTrac Coagulation – Product Status 114
ProTrac Coagulation – Product Description 114
Levisonics Inc Pipeline Products & Ongoing Clinical Trials Overview 115
Blood Coagulation Analyzer – Product Status 115
Blood Coagulation Analyzer – Product Description 115
Pediatric Coagulation Analyzer – Product Status 116
Pediatric Coagulation Analyzer – Product Description 116
Massachusetts General Hospital Pipeline Products & Ongoing Clinical Trials Overview 117
High Throughput Screening Assay – Fibrin-Binding Probes – Product Status 117
High Throughput Screening Assay – Fibrin-Binding Probes – Product Description 117
McMaster University Pipeline Products & Ongoing Clinical Trials Overview 118
aFXa DOAC Assay – Product Status 118
aFXa DOAC Assay – Product Description 118
MiCo BioMed Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 119
Labchip Based Test – Hemoglobin – Product Status 119
Labchip Based Test – Hemoglobin – Product Description 119
Novametics Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 120
Blood Coagulation Diagnostic Assay – Product Status 120
Blood Coagulation Diagnostic Assay – Product Description 120
Ohio State University Pipeline Products & Ongoing Clinical Trials Overview 121
Diagnostic Assay – Thrombotic Thrombocytopenic Purpura – Product Status 121
Diagnostic Assay – Thrombotic Thrombocytopenic Purpura – Product Description 121
Optolane Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 122
Molecular Dx – Thrombosis Panel – Product Status 122
Molecular Dx – Thrombosis Panel – Product Description 122
Pakwa Technologies Pipeline Products & Ongoing Clinical Trials Overview 123
Platelet ActiSense – Product Status 123
Platelet ActiSense – Product Description 123
Perosphere Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 124
PoC Coagulometer – Product Status 124
PoC Coagulometer – Product Description 124
Perosphere Technologies Inc – Ongoing Clinical Trials Overview 125
PoC Coagulometer – Multi-site Study for Evaluation of Clinical Ranges of Whole Blood Clotting Times of Patients on Anticoagulants and Verification of Measurement Precision of Liquid Quality Controls with the Perosphere Technologies’ PoC Coagulometer 126
PortaScience Inc Pipeline Products & Ongoing Clinical Trials Overview 127
PortaPT – Product Status 127
PortaPT – Product Description 127
PortaWBC – Product Status 128
PortaWBC – Product Description 128
Prolight Diagnostics AB Pipeline Products & Ongoing Clinical Trials Overview 129
Point Of Care D-Dimer Test – Product Status 129
Point Of Care D-Dimer Test – Product Description 129
Radiometer Medical ApS Pipeline Products & Ongoing Clinical Trials Overview 130
AQT90 FLEX – APTT Test – Product Status 130
AQT90 FLEX – APTT Test – Product Description 130
AQT90 FLEX – PT-INR Test – Product Status 131
AQT90 FLEX – PT-INR Test – Product Description 131
Randox Laboratories Ltd Pipeline Products & Ongoing Clinical Trials Overview 132
Rx Daytona – D-dimer Assay – Product Status 132
Rx Daytona – D-dimer Assay – Product Description 132
Rx Imola D-dimer Test – Product Status 133
Rx Imola D-dimer Test – Product Description 133
Rappaport Family Institute for Research in the Medical Science Pipeline Products & Ongoing Clinical Trials Overview 134
Diagnostic Kit – Hypercoagulation – Product Status 134
Diagnostic Kit – Hypercoagulation – Product Description 134
Retham Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 135
HITDx – Product Status 135
HITDx – Product Description 135
rHealth Pipeline Products & Ongoing Clinical Trials Overview 136
Diagnostic Test – Thrombocytopenia – Product Status 136
Diagnostic Test – Thrombocytopenia – Product Description 136
Fluorogenic FVIII Assay Kit – Product Status 137
Fluorogenic FVIII Assay Kit – Product Description 137
rHEALTH Coagulation Assay – Product Status 137
rHEALTH Coagulation Assay – Product Description 138
Roche Diagnostics International Ltd Pipeline Products & Ongoing Clinical Trials Overview 139
Cobas t 511 Coagulation Analyzer – dRVVT Confirm Assay – Product Status 139
Cobas t 511 Coagulation Analyzer – dRVVT Confirm Assay – Product Description 140
Cobas t 511 Coagulation Analyzer – dRVVT Screen Assay – Product Status 140
Cobas t 511 Coagulation Analyzer – dRVVT Screen Assay – Product Description 140
Cobas t 711 Coagulation Analyzer – APCr Assay – Product Status 141
Cobas t 711 Coagulation Analyzer – APCr Assay – Product Description 141
Cobas t 711 Coagulation Analyzer – dRVVT Confirm Assay – Product Status 141
Cobas t 711 Coagulation Analyzer – dRVVT Confirm Assay – Product Description 141
Cobas t 711 Coagulation Analyzer – dRVVT Screen Assay – Product Status 142
Cobas t 711 Coagulation Analyzer – dRVVT Screen Assay – Product Description 142
Cobas t 711 Coagulation Analyzer – FXIII Assay – Product Status 142
Cobas t 711 Coagulation Analyzer – FXIII Assay – Product Description 142
Sanguina LLC Pipeline Products & Ongoing Clinical Trials Overview 143
AnemoCheck Home – Product Status 143
AnemoCheck Home – Product Description 143
Sekisui Diagnostics LLC Pipeline Products & Ongoing Clinical Trials Overview 144
Coapresta 2000 Chromogenic Assay – APL – Product Status 144
Coapresta 2000 Chromogenic Assay – APL – Product Description 144
Coapresta 2000 Chromogenic Assay – Heparin – Product Status 145
Coapresta 2000 Chromogenic Assay – Heparin – Product Description 145
Coapresta 2000 Chromogenic Assay – PLG – Product Status 145
Coapresta 2000 Chromogenic Assay – PLG – Product Description 146
Coapresta 2000 Clotting Time Assay – Factor XII – Product Status 146
Coapresta 2000 Clotting Time Assay – Factor XII – Product Description 146
Coapresta 2000 Clotting Time Assay – PC – Product Status 147
Coapresta 2000 Clotting Time Assay – PC – Product Description 147
Coapresta 2000 Latex Turbidimetry Assay – SF – Product Status 147
Coapresta 2000 Latex Turbidimetry Assay – SF – Product Description 148
ShanMukha Innovations Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 149
SickleFind – Product Status 149
SickleFind – Product Description 149
Siemens Healthcare Diagnostics GmbH Pipeline Products & Ongoing Clinical Trials Overview 150
Atellica COAG 360 System – ADP – Product Status 150
Atellica COAG 360 System – ADP – Product Description 151
Atellica COAG 360 System – Arachidonic Acid Reagent – Product Status 151
Atellica COAG 360 System – Arachidonic Acid Reagent – Product Description 151
Atellica COAG 360 System – Berichrom C-1 Inhibitor Assay – Product Status 152
Atellica COAG 360 System – Berichrom C-1 Inhibitor Assay – Product Description 152
Atellica COAG 360 System – Collagen Reagent – Product Status 152
Atellica COAG 360 System – Collagen Reagent – Product Description 153
Atellica COAG 360 System – Epinephrine Reagent – Product Status 153
Atellica COAG 360 System – Epinephrine Reagent – Product Description 153
Atellica COAG 360 System – Lyophilized Platelets Reagent – Product Status 154
Atellica COAG 360 System – Lyophilized Platelets Reagent – Product Description 154
Atellica COAG 360 System – N Antiserum to Human Antithrombin III Assay – Product Status 154
Atellica COAG 360 System – N Antiserum to Human Antithrombin III Assay – Product Description 155
Atellica COAG 360 System – N Antiserum to Human C1 Inhibitor Assay – Product Status 155
Atellica COAG 360 System – N Antiserum to Human C1 Inhibitor Assay – Product Description 155
Atellica COAG 360 System – N Antiserum To Human Fibrinogen Assay – Product Status 155
Atellica COAG 360 System – N Antiserum To Human Fibrinogen Assay – Product Description 156
Atellica COAG 360 System – N Antiserum to Human Plasminogen Assay – Product Status 156
Atellica COAG 360 System – N Antiserum to Human Plasminogen Assay – Product Description 156
Atellica COAG 360 System – Protein S Ac Assay – Product Status 156
Atellica COAG 360 System – Protein S Ac Assay – Product Description 157
Atellica COAG 360 System – Ristocetin Reagent – Product Status 157
Atellica COAG 360 System – Ristocetin Reagent – Product Description 157
Siemens Healthcare Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 158
ADVIA 120 Hematology System – IRF Test – Product Status 158
ADVIA 120 Hematology System – IRF Test – Product Description 159
ADVIA 120 Hematology System – MPC Test – Product Status 159
ADVIA 120 Hematology System – MPC Test – Product Description 159
ADVIA 120 Hematology System – MPM Test – Product Status 159
ADVIA 120 Hematology System – MPM Test – Product Description 160
ADVIA 2120 Hematology System – MPC Test – Product Status 160
ADVIA 2120 Hematology System – MPC Test – Product Description 160
ADVIA 2120 Hematology System – MPM Test – Product Status 161
ADVIA 2120 Hematology System – MPM Test – Product Description 161
ADVIA 2120i Hematology System – MPC Test – Product Status 161
ADVIA 2120i Hematology System – MPC Test – Product Description 162
ADVIA 2120i Hematology System – MPM Test – Product Status 162
ADVIA 2120i Hematology System – MPM Test – Product Description 162
ADVIA Centaur XP Immunoassay System – D-Dimer Assay – Product Status 163
ADVIA Centaur XP Immunoassay System – D-Dimer Assay – Product Description 163
Sysmex CS-2000i System – Multifibren U Assay – Product Status 163
Sysmex CS-2000i System – Multifibren U Assay – Product Description 164
Sysmex CS-2100i System – Multifibren U Assay – Product Status 164
Sysmex CS-2100i System – Multifibren U Assay – Product Description 164
Sysmex CS-2500 System – Von Willebrand Factor Test – Product Status 165
Sysmex CS-2500 System – Von Willebrand Factor Test – Product Description 165
Sysmex CS-5100 System – Von Willebrand Factor Assay – Product Status 165
Sysmex CS-5100 System – Von Willebrand Factor Assay – Product Description 166
Siemens Healthineers AG Pipeline Products & Ongoing Clinical Trials Overview 167
IMMULITE 1000 System – D-Dimer Assay – Product Status 167
IMMULITE 1000 System – D-Dimer Assay – Product Description 168
IMMULITE 1000 System – Turbo D-Dimer Assay – Product Status 168
IMMULITE 1000 System – Turbo D-Dimer Assay – Product Description 168
Sysmex CA-1500 – Innovance VWF Activity Assay – Product Status 168
Sysmex CA-1500 – Innovance VWF Activity Assay – Product Description 169
Sysmex CA-1500 – PT Multicalibrators Assay – Product Status 169
Sysmex CA-1500 – PT Multicalibrators Assay – Product Description 169
Sysmex CA-530 – PT Multicalibrators Assay – Product Status 170
Sysmex CA-530 – PT Multicalibrators Assay – Product Description 170
Sysmex CA-560 – Innovance VWF Activity Assay – Product Status 170
Sysmex CA-560 – Innovance VWF Activity Assay – Product Description 171
Sysmex CA-560 – PT Multicalibrators Assay – Product Status 171
Sysmex CA-560 – PT Multicalibrators Assay – Product Description 171
Sysmex CA-7000 – Innovance VWF Activity Assay – Product Status 172
Sysmex CA-7000 – Innovance VWF Activity Assay – Product Description 172
Sysmex CA-7000 – PT Multicalibrators Assay – Product Status 172
Sysmex CA-7000 – PT Multicalibrators Assay – Product Description 173
Silver Lake Research Corporation Pipeline Products & Ongoing Clinical Trials Overview 174
HemoTypeBT – Product Status 174
HemoTypeBT – Product Description 174
SonoMedix, Inc. Pipeline Products & Ongoing Clinical Trials Overview 175
NanoAcoustic Blood Analyzer – Product Status 175
NanoAcoustic Blood Analyzer – Product Description 175
SpinChip Diagnostics AS Pipeline Products & Ongoing Clinical Trials Overview 176
D-Dimer Assay – Product Status 176
D-Dimer Assay – Product Description 176
SpinChip – APTT Assay – Product Status 177
SpinChip – APTT Assay – Product Description 177
SpinChip – PT Assay – Product Status 177
SpinChip – PT Assay – Product Description 178
SQI Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 179
IgXPlex – HIT Assay – Product Status 179
IgXPlex – HIT Assay – Product Description 179
Stichting Lygature Pipeline Products & Ongoing Clinical Trials Overview 180
Acute Marker Assay – Product Status 180
Acute Marker Assay – Product Description 180
Blood Capacity Assay – Product Status 181
Blood Capacity Assay – Product Description 181
T2 Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview 182
T2HemoStat – Product Status 182
T2HemoStat – Product Description 182
T2MR Assay – Platelet Function Testing – Product Status 183
T2MR Assay – Platelet Function Testing – Product Description 183
The Binding Site Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 184
Optilite – Fibrinogen Assay – Product Status 184
Optilite – Fibrinogen Assay – Product Description 184
ThromboTherapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 185
tPA-Challenge Assay – Product Status 185
tPA-Challenge Assay – Product Description 185
Tosoh Bioscience Inc Pipeline Products & Ongoing Clinical Trials Overview 186
AIA-1800 – D-dimer Test – Product Status 186
AIA-1800 – D-dimer Test – Product Description 186
AIA-600 II – D-dimer Test – Product Status 187
AIA-600 II – D-dimer Test – Product Description 187
Transfusion & Transplantation Technologies (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 188
Aegis – DIC Assay – Product Status 188
Aegis – DIC Assay – Product Description 188
Universal Biosensors Inc Pipeline Products & Ongoing Clinical Trials Overview 189
Diagnostic Assay – Coagulation – Product Status 189
Diagnostic Assay – Coagulation – Product Description 189
Handheld POC Coagulation Testing System – Ambulatory Care – Product Status 190
Handheld POC Coagulation Testing System – Ambulatory Care – Product Description 190
Immunoassay Test – D-Dimer – Product Status 190
Immunoassay Test – D-Dimer – Product Description 191
Xprecia Prime – Product Status 191
Xprecia Prime – Product Description 191
University of Burdwan Pipeline Products & Ongoing Clinical Trials Overview 192
Hemoglobinopathy- Thalassemia Carrier Screening Kit – Product Status 192
Hemoglobinopathy- Thalassemia Carrier Screening Kit – Product Description 192
University of California Irvine Pipeline Products & Ongoing Clinical Trials Overview 193
Diagnostic Tool – Blood Coagulation – Product Status 193
Diagnostic Tool – Blood Coagulation – Product Description 193
University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 194
Thrombelastography Assay – Product Status 194
Thrombelastography Assay – Product Description 194
University of Florida Pipeline Products & Ongoing Clinical Trials Overview 195
Hemoglobin Measurement Device – Product Status 195
Hemoglobin Measurement Device – Product Description 195
University of Melbourne Pipeline Products & Ongoing Clinical Trials Overview 196
Thrombin Generation Assay – Product Status 196
Thrombin Generation Assay – Product Description 196
University of Michigan Pipeline Products & Ongoing Clinical Trials Overview 197
mHemoRetractoMeter – Product Status 197
mHemoRetractoMeter – Product Description 197
University of Texas Health Science Center at Houston Pipeline Products & Ongoing Clinical Trials Overview 198
MEMS Based Blood Coagulometer – Product Status 198
MEMS Based Blood Coagulometer – Product Description 198
University of Vermont Pipeline Products & Ongoing Clinical Trials Overview 199
Diagnostic Assay – Hemophilia – Product Status 199
Diagnostic Assay – Hemophilia – Product Description 199
University of Washington Pipeline Products & Ongoing Clinical Trials Overview 200
Blood-Clotting Test – Product Status 200
Blood-Clotting Test – Product Description 200
VitaMe Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 201
Iron.Scan – Product Status 201
Iron.Scan – Product Description 201
Glossary 290
![]()