Homozygous Familial Hypercholesterolemia (HoFH) – Global Clinical Trials Review, H2, 2021
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
GlobalData’s clinical trial report provides an overview of the homozygous familial hypercholesterolemia (hofh) clinical trials scenario. This report provides top-line data relating to the clinical trials on homozygous familial hypercholesterolemia. The report includes an overview of trial numbers and their average enrolment in top countries conducted across the globe. The report offers coverage of disease clinical trials by region, country (G7 & E7), phase, trial status, end points status, and sponsor type. The report also provides prominent drugs for in-progress trials (based on the number of ongoing trials). The report enhances the decision-making capabilities and helps to create effective counterstrategies to gain a competitive advantage.
The homozygous familial hypercholesterolemia (hofh) clinical trial report consists of 419 trials. Trials conducted by companies in Phase IV, Phase III, Phase II, Phase I, and Phase 0 stand at 14, 32, 29, 319, and 2 respectively. Similarly, the trials with different status compile of Completed – 306, Ongoing – 30, Planned – 40, Suspended – 1, Terminated – 5, and Withdrawn – 37. Out of 306 completed trials 203 trials have achieved endpoint.
What are the market dynamics of the global homozygous familial hypercholesterolemia therapeutics clinical trials sector?
The number of homozygous familial hypercholesterolemia clinical trials conducted globally has increased by 93% for the period 2016-2020. The average number of patients enrolled was highest in the year 2016. Out of the total clinical trials conducted, over 90% of trials have been sponsored by the company and more than 5% by institutions. As of November 2021, there were 30 clinical trials in progress and 306 trials were completed. The trials that were terminated/suspended or withdrawn accounted for 43. This was due to the lack of efficacy, safety, and lack of accrual of subjects. Out of 306 completed trials, 212 trials have results and 203 (96%) of trials reached endpoints.
What are the top regions and countries in the global homozygous familial hypercholesterolemia therapeutics clinical trials sector?
In total there were 419 clinical trials conducted on homozygous familial hypercholesterolemia, as of November 2021, of these 138 clinical trials were in Asia-Pacific. More than 30% of the clinical trials were conducted in Asia-Pacific. During the same period, Germany has the highest average patient enrollment of homozygous familial hypercholesterolemia clinical trials.
Asia-Pacific: In the Asia-Pacific region, China has the highest number of homozygous familial hypercholesterolemia clinical trials, as of November 2021, followed by Thailand, Japan, South Korea, and India.
Europe: Among the European countries, Russia has the highest number of homozygous familial hypercholesterolemia clinical trials, as of November 2021, followed by the Netherlands, Italy, Germany, and France.
North America: In the country-wise analysis, the US has the highest number of homozygous familial hypercholesterolemia clinical trials, as of November 2021, followed by Canada, and Mexico.
The Middle East and Africa: South Africa has the highest number of homozygous familial hypercholesterolemia (hofh) clinical trials followed by Israel, Lebanon, Iran, and Jordan which are the leading countries in the region based on homozygous familial hypercholesterolemia clinical trials.
Central and South America: Brazil has the highest number of homozygous familial hypercholesterolemia clinical trials in the region followed by Argentina and Colombia.
G7 countries: Among the G7 (the US, the UK, Germany, France, Italy, Canada, and Japan) countries, Canada has the highest proportion of Homozygous familial hypercholesterolemia to metabolic disorders clinical trials as of November 2021. In total there were 159 clinical trials conducted on homozygous familial hypercholesterolemia, as of November 2021 in G7 Countries, of these 51 clinical trials were in the US. During the same period, there were 13 clinical trials in progress and 58 trials are completed. The trials that are terminated/suspended or withdrawn accounted for 11. This was due to the lack of efficacy, safety, and lack of accrual of subjects.
E7 countries: Among the E7 (Brazil, Russia, India, China, Mexico, Turkey, and Indonesia) countries, Russia has the highest proportion of Homozygous familial hypercholesterolemia to metabolic disorders clinical trials as of November 2021. During the same period, in total there were 130 clinical trials conducted on homozygous familial hypercholesterolemia of these 53 clinical trials were in China. There were 20 clinical trials in progress and 76 trials were completed. The trials that were terminated/suspended or withdrawn accounted for 11. As of November 2021, the majority of the trials were successfully completed.
Global homozygous familial hypercholesterolemia therapeutics clinical trials sector, by regions
For more regional insights, download a free report sample
Which are the key companies in the global homozygous familial hypercholesterolemia therapeutics clinical trials sector?
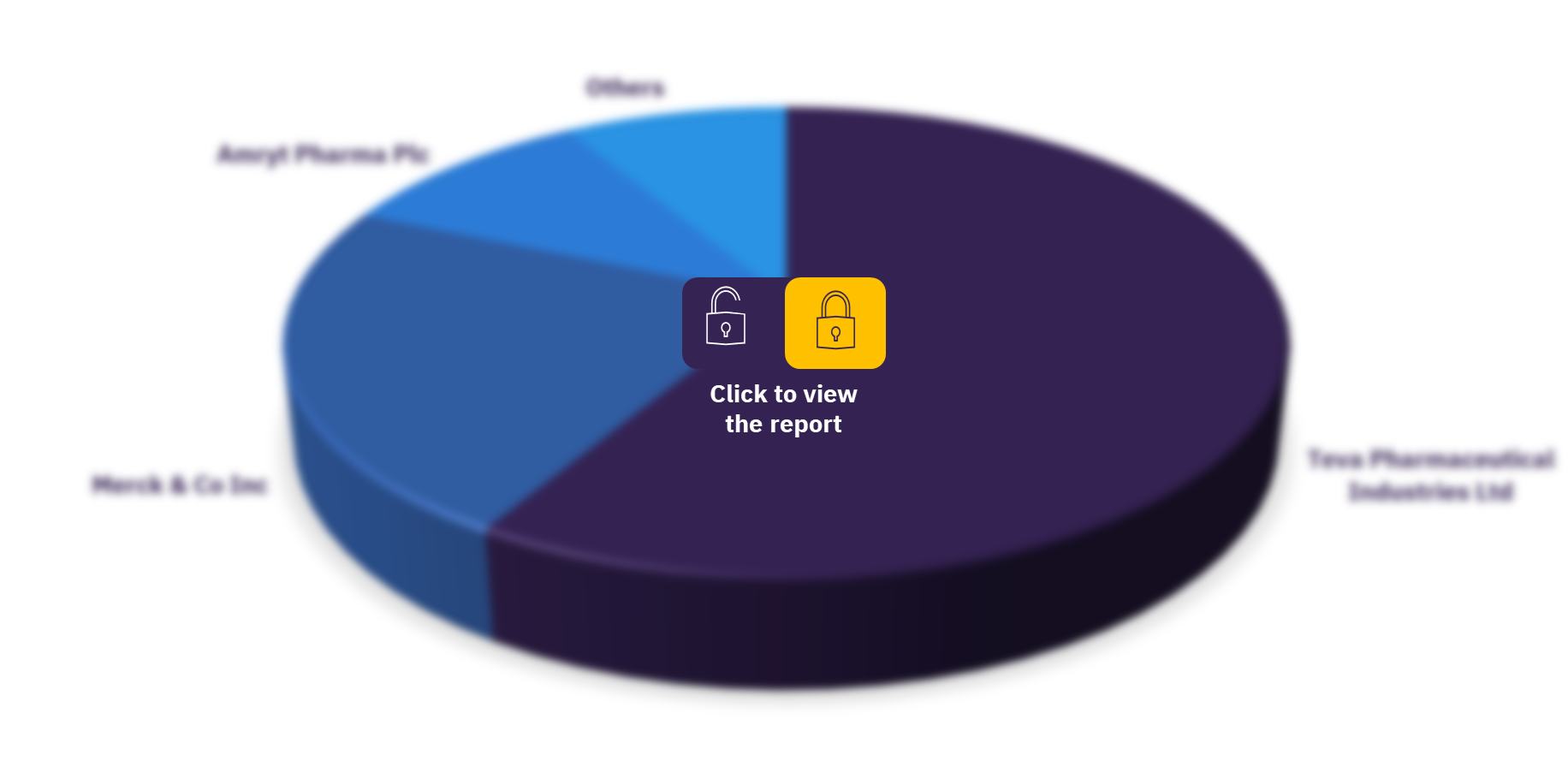
As of November 2021, Teva Pharmaceutical Industries Ltd has conducted the highest number of homozygous familial hypercholesterolemia clinical trials, followed by Merck & Co Inc, and Amryt Pharma Plc. Other major companies in the sector are Dr. Reddy’s Laboratories Ltd, International Bio Service Co Ltd, Novartis AG, Amgen Inc, Krka dd Novo Mesto, Bio-Innova & Synchron Co Ltd, and Madrigal Pharmaceuticals Inc.
Global homozygous familial hypercholesterolemia therapeutics clinical trials sector, by key companies
To know more about key companies, download a free report sample
Market report scope
|
Key countries |
China, the US, Russia, Canada, Thailand, the Netherlands, Japan, Italy, South Korea, and Germany |
| Key companies | Teva Pharmaceutical Industries Ltd, Merck & Co Inc, Amryt Pharma Plc, Dr. Reddy’s Laboratories Ltd, International Bio Service Co Ltd, Novartis AG, Amgen Inc, Krka dd Novo Mesto, Bio-Innova & Synchron Co Ltd, and Madrigal Pharmaceuticals Inc. |
Scope
- The report provides a snapshot of the global clinical trials landscape.
- Report provides top-level data related to the clinical trials by region, country (G7 & E7), trial status, trial phase, sponsor type, and endpoint status.
- The report reviews the top companies involved and enlists all trials (Trial title, Phase, and Status) pertaining to the company.
- The report provides all the unaccomplished trials (terminated, suspended, and withdrawn) with the reason for unaccomplishment.
- The report provides enrollment trends for the past five years.
- Report provides the latest news for the past three months.
Reasons to Buy
- Assists in formulating key business strategies with regard to investment.
- Helps in identifying prominent locations for conducting clinical trials which saves time and cost.
- Provides top-level analysis of the global clinical trials market which helps in identifying key business opportunities.
- Supports understanding of trials count and enrolment trends by country in the global therapeutics market.
- Aids in interpreting the success rates of clinical trials by providing a comparative scenario of completed and uncompleted (terminated, suspended, or withdrawn) trials.
- Facilitates clinical trial assessment of the indication on a global, regional, and country level.
Merck & Co Inc
Amryt Pharma Plc
Dr. Reddy's Laboratories Ltd
International Bio Service Co Ltd
Novartis AG
Amgen Inc
Krka dd Novo Mesto
Bio-Innova & Synchron Co Ltd
Madrigal Pharmaceuticals Inc
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key countries in the global homozygous familial hypercholesterolemia therapeutics clinical trials sector?
The key countries in the global homozygous familial hypercholesterolemia therapeutics clinical trials sector are China, the US, Russia, Canada, Thailand, the Netherlands, Japan, Italy, South Korea, and Germany.
-
Which are the key companies in the global homozygous familial hypercholesterolemia therapeutics clinical trials sector?
The key companies in the global homozygous familial hypercholesterolemia therapeutics clinical trials sector are Teva Pharmaceutical Industries Ltd, Merck & Co Inc, Amryt Pharma Plc, Dr. Reddy’s Laboratories Ltd, International Bio Service Co Ltd, Novartis AG, Amgen Inc, Krka dd Novo Mesto, Bio-Innova & Synchron Co Ltd, and Madrigal Pharmaceuticals Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Metabolic Disorders reports