Huntingtin (Huntington Disease Protein or HTT) Drugs in Development by Therapy Areas and Indications, Stages, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Huntingtin Pipeline Products Market Report Overview
Huntingtin (Huntington Disease Protein or HTT) upregulates the expression of brain-derived neurotrophic factor (BDNF) at the transcription level. Huntingtin is primarily associated with vesicles and microtubules. These indicate an important role in cytoskeletal anchoring or transport of mitochondria.
The Huntingtin pipeline market research report provides comprehensive information on the Huntingtin (Huntington Disease Protein or HTT) targeted therapeutics, complete with analysis by indications, stage of development, mechanism of action (MoA), route of administration (RoA), and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history, the latest news as well as press releases. Additionally, the report provides an overview of key players involved in Huntingtin (Huntington Disease Protein or HTT) targeted therapeutics development with respective active and dormant or discontinued projects.
Key Therapy Areas in the Huntingtin Pipeline Products Market
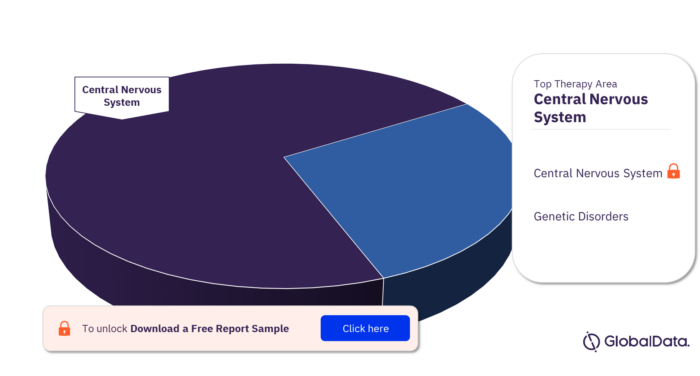
The key therapy areas in the Huntingtin pipeline products market are central nervous system and genetic disorders.
Huntingtin Pipeline Products Market Analysis by Therapy Areas
For more therapy area insights, download a free report sample
Key Mechanisms of Action in the Huntingtin Pipeline Products Market
The key mechanism of action in the Huntingtin pipeline products market is Huntingtin Inhibitor.
Key Routes of Administration in the Huntingtin Pipeline Products Market
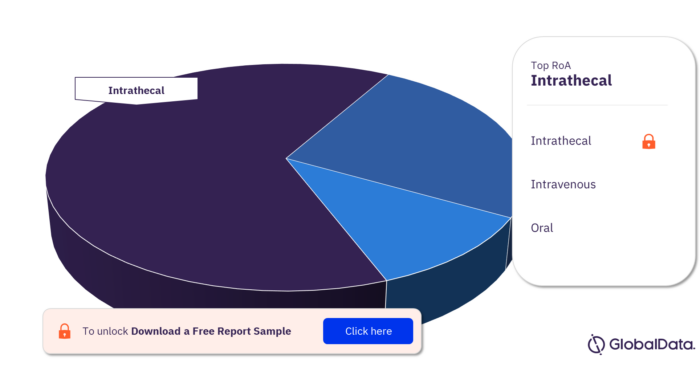
The key routes of administration in the Huntingtin pipeline products market are intrathecal, intravenous, and oral.
Huntingtin Pipeline Products Market Analysis by Routes of Administration
For more routes of administration insights, download a free report sample
Key Molecule Types in the Huntingtin Pipeline Products Market
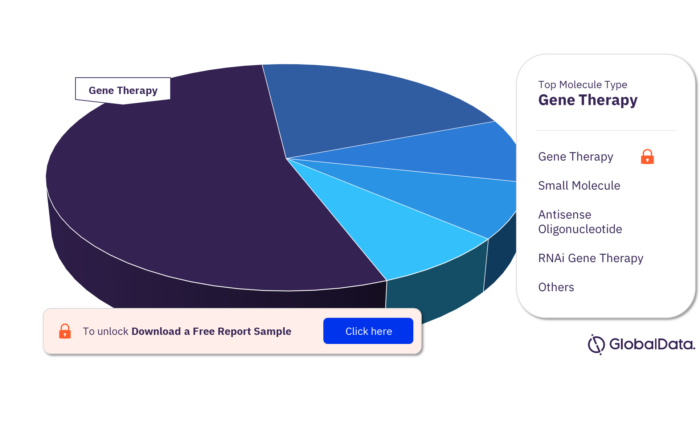
The key molecule types in the Huntingtin pipeline products market are gene therapy, small molecule, antisense oligonucleotide, RNAi gene therapy, antisense RNAi oligonucleotide, monoclonal antibody, synthetic peptide, conjugate vaccine, oligonucleotide, and recombinant peptide.
Huntingtin Pipeline Products Market Analysis by Molecule Types
For more molecule type insights, download a free report sample
Major Companies in the Huntingtin Pipeline Products Market
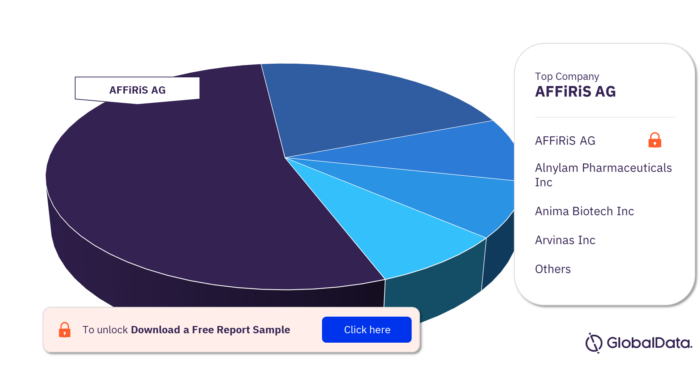
Some of the major companies in the Huntingtin pipeline products market are AFFiRiS AG, Alnylam Pharmaceuticals Inc, Anima Biotech Inc, Arvinas Inc, Atalanta Therapeutics Inc, Ceptur Therapeutics Inc, Dystrogen Therapeutics SA, Enzerna Biosciences LLC, F. Hoffmann-La Roche Ltd, and Locanabio Inc.
Huntingtin Pipeline Products Market Analysis by Companies
To know more about major companies, download a free report sample
Huntingtin Pipeline Products Market Report Overview
| Key Therapy Areas | Central Nervous System and Genetic Disorders |
| Key Mechanisms of Action | Huntingtin Inhibitor |
| Key Routes of Administration | Intrathecal, Intravenous, and Oral |
| Key Molecule Type | Gene Therapy, Small Molecule, Antisense Oligonucleotide, RNAi Gene Therapy, Antisense RNAi Oligonucleotide, Monoclonal Antibody, Synthetic Peptide, Conjugate Vaccine, Oligonucleotide, and Recombinant Peptide |
| Major Companies | AFFiRiS AG, Alnylam Pharmaceuticals Inc, Anima Biotech Inc, Arvinas Inc, Atalanta Therapeutics Inc, Ceptur Therapeutics Inc, Dystrogen Therapeutics SA, Enzerna Biosciences LLC, F. Hoffmann-La Roche Ltd, and Locanabio Inc |
Scope
- The report provides a snapshot of the global therapeutic landscape for Huntingtin (Huntington Disease Protein or HTT)
- The report reviews Huntingtin (Huntington Disease Protein or HTT) targeted therapeutics under development by companies and universities/research institutes based on information derived from company and industry-specific sources
- The report covers pipeline products based on various stages of development ranging from pre-registration till discovery and undisclosed stages
- The report features descriptive drug profiles for the pipeline products which includes, product description, descriptive MoA, R&D brief, licensing and collaboration details & other developmental activities
- The report reviews key players involved in Huntingtin (Huntington Disease Protein or HTT) targeted therapeutics and enlists all their major and minor projects
- The report assesses Huntingtin (Huntington Disease Protein or HTT) targeted therapeutics based on mechanism of action (MoA), route of administration (RoA), and molecule type
- The report summarizes all the dormant and discontinued pipeline projects
- The report reviews the latest news and deals related to Huntingtin (Huntington Disease Protein or HTT) targeted therapeutics
Reasons to Buy
- Gain strategically significant competitor information, analysis, and insights to formulate effective R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage
- Identify and understand the targeted therapy areas and indications for Huntingtin (Huntington Disease Protein or HTT)
- Identify the use of drugs for target identification and drug repurposing
- Identify potential new clients or partners in the target demographic
- Develop strategic initiatives by understanding the focus areas of leading companies
- Plan mergers and acquisitions effectively by identifying key players and their most promising pipeline therapeutics
- Devise corrective measures for pipeline projects by understanding Huntingtin (Huntington Disease Protein or HTT) development landscape
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope
Alnylam Pharmaceuticals Inc
Anima Biotech Inc
Arvinas Inc
Atalanta Therapeutics Inc
Ceptur Therapeutics Inc
Dystrogen Therapeutics SA
Enzerna Biosciences LLC
F. Hoffmann-La Roche Ltd
Locanabio Inc
Neurimmune Holding AG
Novartis AG
Ophidion Inc
Origami Therapeutics Inc
Passage Bio Inc
Primary Peptides Inc
PTC Therapeutics Inc
reMYND NV
ResQ Biotech
Takeda Pharmaceutical Co Ltd
UniQure NV
Voyager Therapeutics Inc
Vybion Inc
Wave Life Sciences Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key therapy areas in the Huntingtin pipeline products market?
The key therapy areas in the Huntingtin pipeline products market are central nervous system and genetic disorders.
-
What are the key mechanisms of action in the Huntingtin pipeline products market?
The key mechanism of action in the Huntingtin pipeline products market is Huntingtin Inhibitor.
-
What are the key routes of administration in the Huntingtin pipeline products market?
The key routes of administration in the Huntingtin pipeline products market are intrathecal, intravenous, and oral.
-
What are the key molecule types in the Huntingtin pipeline products market?
The key molecule types in the Huntingtin pipeline products market are gene therapy, small molecule, antisense oligonucleotide, RNAi gene therapy, antisense RNAi oligonucleotide, monoclonal antibody, synthetic peptide, conjugate vaccine, oligonucleotide, and recombinant peptide.
-
Which are the major companies in the Huntingtin pipeline products market?
Some of the major companies in the Huntingtin pipeline products market are AFFiRiS AG, Alnylam Pharmaceuticals Inc, Anima Biotech Inc, Arvinas Inc, Atalanta Therapeutics Inc, Ceptur Therapeutics Inc, Dystrogen Therapeutics SA, Enzerna Biosciences LLC, F. Hoffmann-La Roche Ltd, and Locanabio Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Other Diseases reports