Hydrogels Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Hydrogels Pipeline Market Report Overview
Hydrogels include dressings that consist of 96% water with a matrix of insoluble polymers embedded in it. The former helps to maintain a moist wound environment while the latter facilitates absorption of wound exudates. These dressings are available in the form of tubes and sheets and both of them are tracked under this segment. Hydrogels are segmented into antimicrobial and non-antimicrobial hydrogel dressings.
The Hydrogels pipeline market research report provides comprehensive information about the Hydrogels pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Territories | · The US
· China · Europe · Global · Australia |
| Key Segments | · Antimicrobial Hydrogel Dressings
· Non-Antimicrobial Hydrogel Dressings |
| Key Regulatory Paths | · 510(k)
· NMPA · ICAC · TGA · Ninsho |
| Leading Companies | · 3QMatrix, Inc. (Inactive)
· Abeona Therapeutics Inc · AgeX Therapeutics Inc · Alafair Biosciences Inc · Albert Ludwigs University of Freiburg |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Hydrogels Pipeline Market Segmentation by Segments
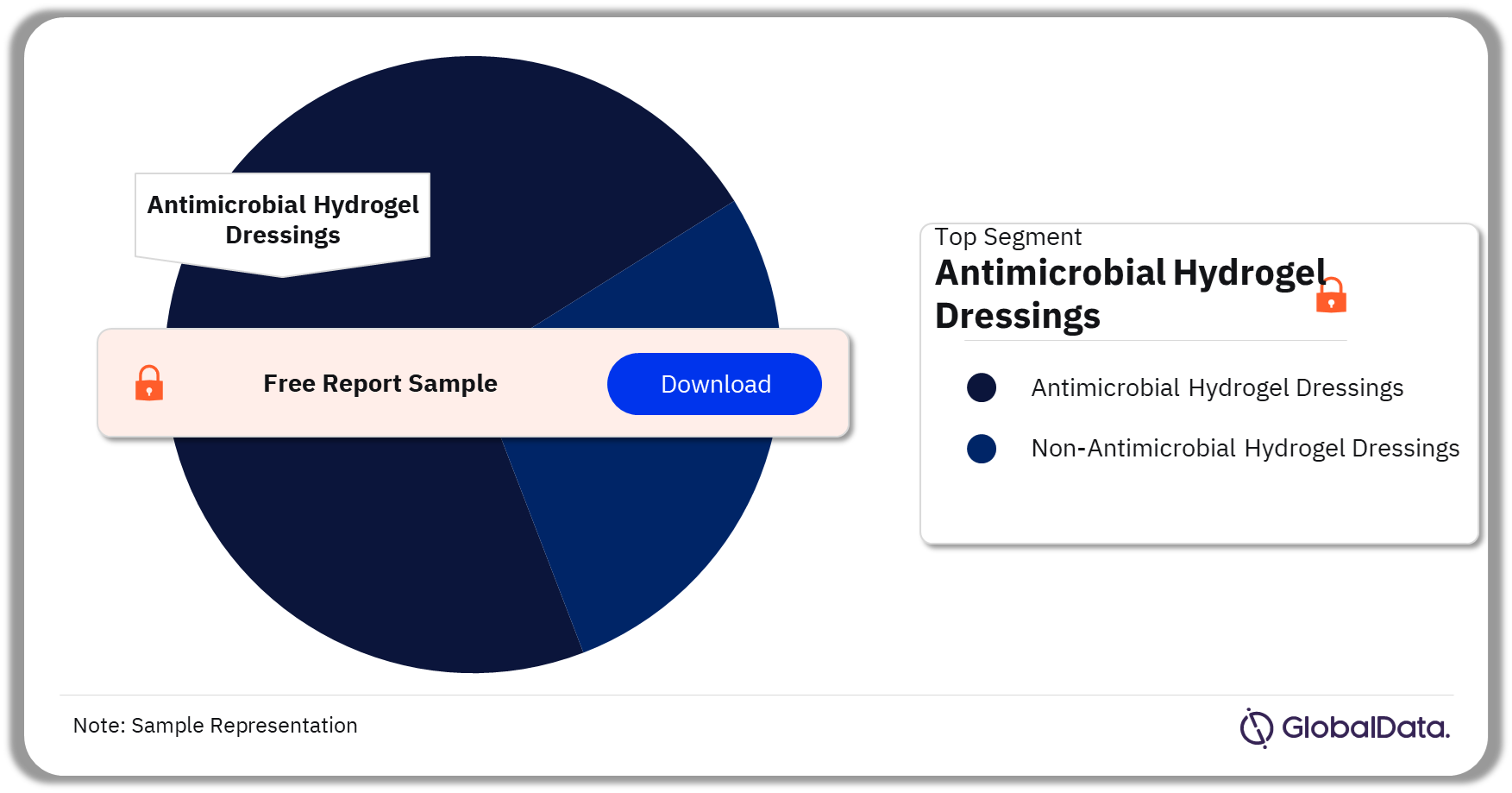
Some of the key segments in the Hydrogels market are Antimicrobial Hydrogel Dressings and Non-Antimicrobial Hydrogel Dressings. As of November 2023, Antimicrobial Hydrogel Dressings has the highest number of pipeline products.
Antimicrobial Hydrogel Dressings are dressings impregnated with an agent that inhibits microbial growth. Non-Antimicrobial Hydrogel Dressings are dressings devoid of antimicrobial activity due to the absence of antimicrobial agents.
Hydrogels Pipeline Market Analysis by Segments, 2023 (%)
Buy the Full Report for More Segments Insights into the Hydrogels Pipeline Market
Hydrogels Pipeline Market Segmentation by Territories
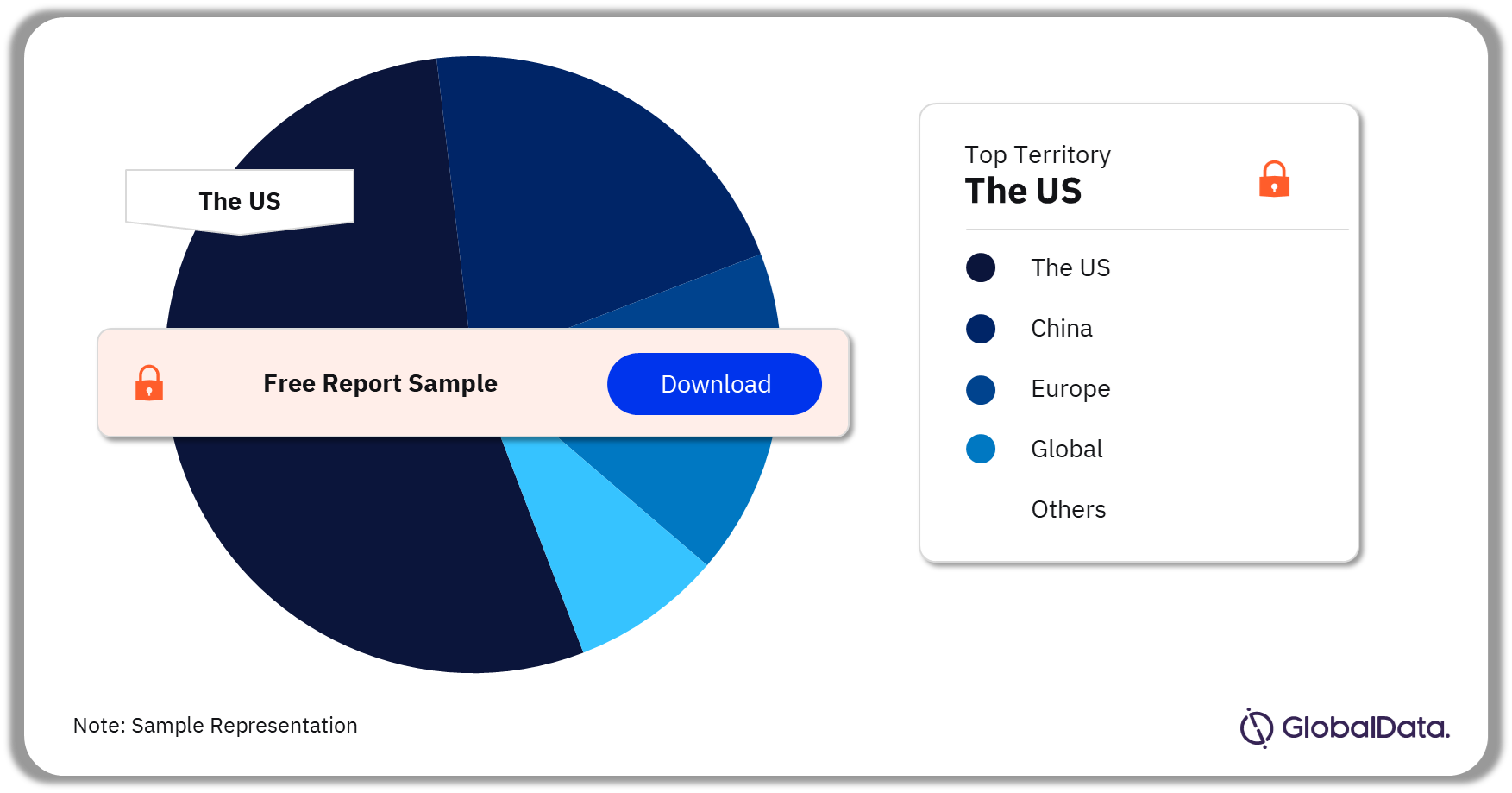
Some of the key territories in the Hydrogels market are the US, China, Europe, Global, and Australia among others. As of November 2023, the US has the highest number of pipeline products.
Hydrogels Pipeline Market Analysis by Territories, 2023 (%)
Buy the Full Report for More Territory Insights into the Hydrogels Pipeline Market
Hydrogels Pipeline Market Segmentation by Regulatory Paths
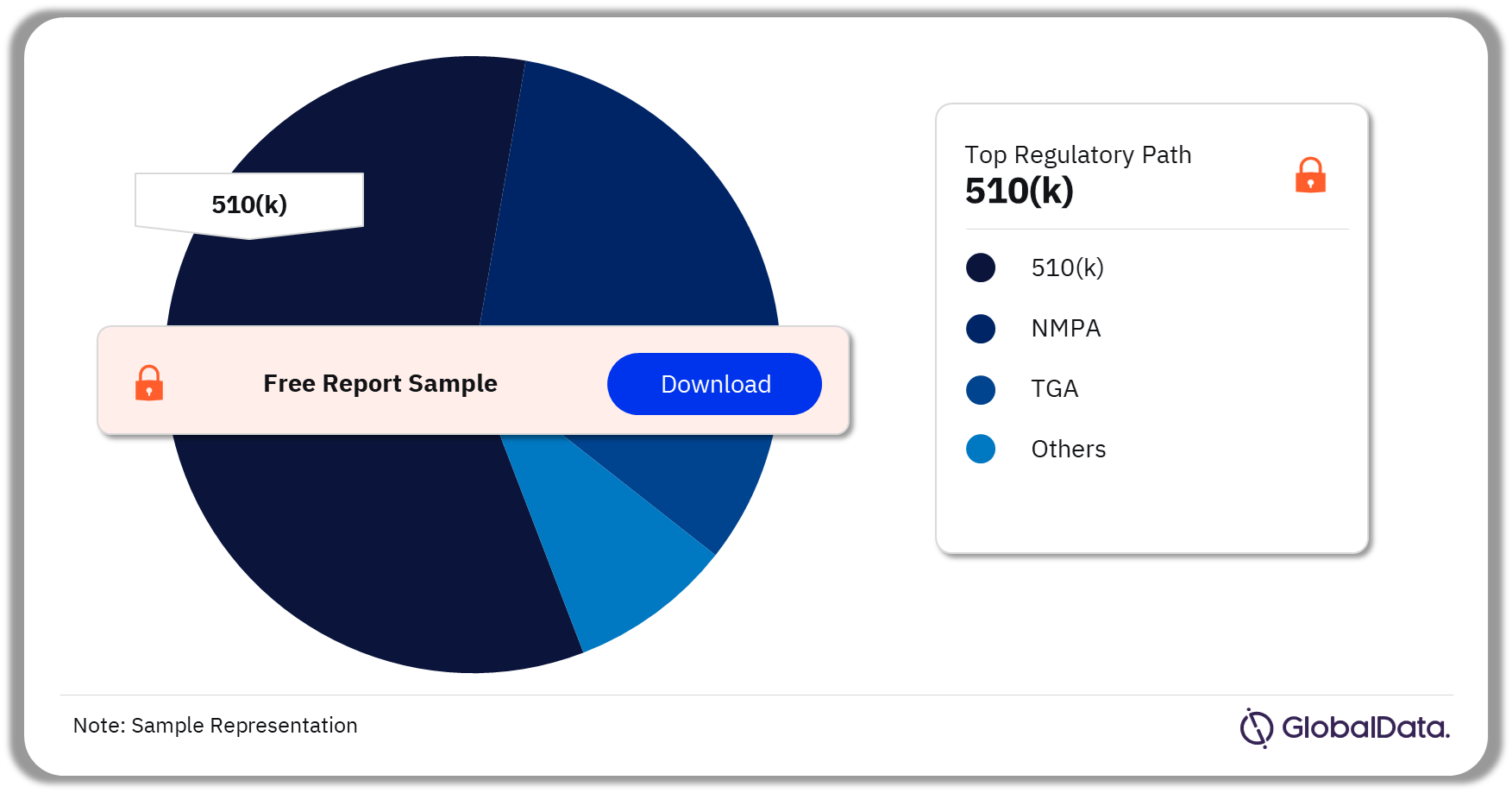
Some of the key regulatory paths in the Hydrogels pipeline market are 510(k), NMPA, ICAC, TGA, and Ninsho. As of November 2023, 510(k) is the most followed pathway for pipeline products in the market.
Hydrogels Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy the Full Report for More Regulatory Path Insights into the Hydrogels Pipeline Market
Hydrogels Pipeline Market – Competitive Landscape
Some of the key companies in the Hydrogels pipeline market are:
- 3QMatrix, Inc. (Inactive)
- Abeona Therapeutics Inc
- AgeX Therapeutics Inc
- Alafair Biosciences Inc
- Albert Ludwigs University of Freiburg
Abeona Therapeutics Inc (Abeona). It, formerly PlasmaTech Biopharmaceuticals Inc., is a biopharmaceutical company that develops and delivers gene therapy and plasma-based products for severe life-threatening rare diseases. The company’s lead programs include EB-101, ABO-101 and ABO-102 adeno-associated virus. Abeona develops EB-101 for recessive dystrophic epidermolysis bullosa (RDEB), EB-201 for epidermolysis bullosa (EB), ABO-202 (AAV-CLN1) gene therapy for the treatment of infantile Batten disease (INCL), ABO-201 (AAV-CLN3) gene therapy for Juvenile Batten disease (JNCL) and more. The company has partnerships for the commercialization of its products in the US, European Union, Switzerland, Norway, Iceland, Lichtenstein, Australia, New Zealand, China and South Korea. Abeona is headquartered in New York City, the US.
Hydrogels Pipeline Market Analysis by Companies, 2023
Buy the Full Report for More Company Insights into the Hydrogels Pipeline Market
Segments Covered in the Report
Hydrogels Pipeline Market Segments Outlook
- Antimicrobial Hydrogel Dressings
- Non-Antimicrobial Hydrogel Dressings
Hydrogels Pipeline Market Territories Outlook
- The US
- China
- Europe
- Global
- Australia
Hydrogels Pipeline Market Regulatory Paths Outlook
- 510(k)
- NMPA
- ICAC
- TGA
- NINSHO
Scope
This report provides:
- Extensive coverage of the Hydrogels under development.
- Review details of major pipeline products which include product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in pipeline product development and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Provides key clinical trial data related to ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to – _x000D_
– Formulate significant competitor information, analysis, and insights to improve R&D strategies_x000D_
– Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage_x000D_
– Identify and understand important and diverse types of Hydrogels under development_x000D_
– Develop market-entry and market expansion strategies_x000D_
– Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline_x000D_
– In-depth analysis of the product’s current stage of development, territory and estimated launch date
Abeona Therapeutics Inc
AgeX Therapeutics Inc
Alafair Biosciences Inc
Albert Ludwigs University of Freiburg
Amend Surgical, Inc.
Angiotech Pharmaceuticals Inc
AXXO Im- und Export GmbH
BioCure Inc
Biomendics LLC
Brigham and Women's Hospital
CelaCare Technologies LLC
Ceramicos Para Aplicacoes Medicas SA
Chi2Gel Ltd.
CorMedix Inc
Drexel University
Edixomed Ltd
Exogenus Therapeutics SA
Flexion Therapeutics Inc
Gel4Med Inc
Gelesis Inc
GelSana Therapeutics Inc
HyperBranch Medical Technology Inc
Icahn School of Medicine at Mount Sinai
In2cure AB
Indian Institute of Technology, Kanpur
Institute of Nano Science and Technology
Ionic Pharmaceuticals LLC
IPI Singapore
Jenarron Therapeutics Ltd
Johns Hopkins University
Kane Biotech Inc
Keratin Biosciences Inc
Magle Chemoswed AB
McMaster University
Missouri University of Science and Technology
Neotherix Ltd
Northwestern University
Novyx Technologies LLC
O2 Regentech LLC
Ohio State University
Orla Protein Technologies Ltd
Piramal Enterprises Ltd
Plurogen Therapeutics Inc (Inactive)
Queensland University of Technology
Quick-Med Technologies Inc
Quthero Inc
Regentys Corp
Scarless Laboratories Inc
SolasCure Ltd
Sonoma Pharmaceuticals Inc
Spanish National Research Council
Squid Healthcare Inc
Stanford University
Sunogel Biotechnologies Inc
SynDermix AG
Syracuse Biolabs Inc
Syracuse University
Tectum
Tel Aviv Sourasky Medical Center
Tel Aviv University
Texas Medical Center
Tokyo University of Science
University of California San Diego
University of Florida
University of Illinois at Chicago
University of Pennsylvania
University of Queensland
University of Wisconsin Madison
Ventrix Inc
VTT Technical Research Centre of Finland Ltd
Washington University in St Louis
Zeomedix, LLC
Table of Contents
Table
Figures
Frequently asked questions
-
Which territory has the highest number of pipeline products in the Hydrogels pipeline market?
As of November 2023, the US has the highest number of products in the Hydrogels pipeline market.
-
Which segment has the highest number of pipeline products in the Hydrogels pipeline market?
As of November 2023, Antimicrobial Hydrogel Dressings has the highest number of pipeline products.
-
Which is the most followed regulatory pathway in the Hydrogels pipeline market?
As of November 2023, 510(k) is the most followed pathway for pipeline products in the Hydrogels market.
-
Who are the major companies operating in the Hydrogels pipeline market?
Some of the key companies in the Hydrogels pipeline market are 3QMatrix, Inc. (Inactive), Abeona Therapeutics Inc, AgeX Therapeutics Inc, Alafair Biosciences Inc, and Albert Ludwigs University of Freiburg among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.