1 Table of Contents 3
|1.1 List of Tables 8
|1.2 List of Figures 12
2 Introduction 13
2.1 Immunochemistry Analyzers Overview 13
3 Products under Development 14
3.1 Immunochemistry Analyzers – Pipeline Products by Stage of Development 14
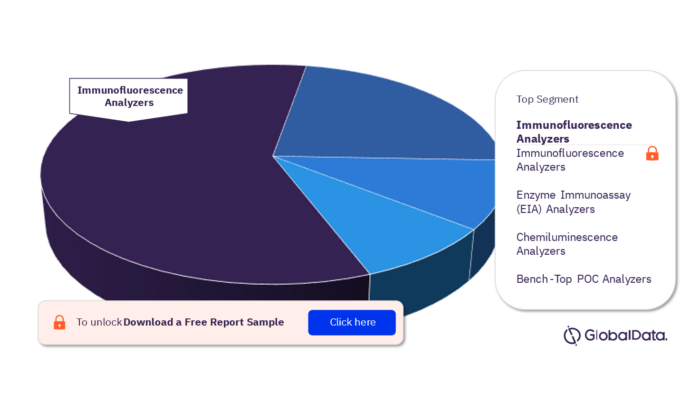
3.2 Immunochemistry Analyzers – Pipeline Products by Segment 15
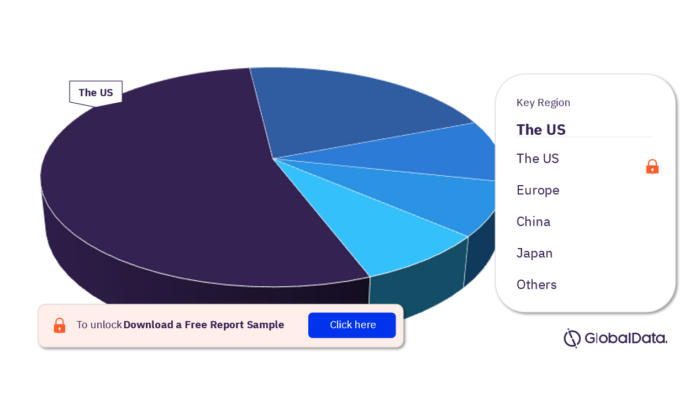
3.3 Immunochemistry Analyzers – Pipeline Products by Territory 16
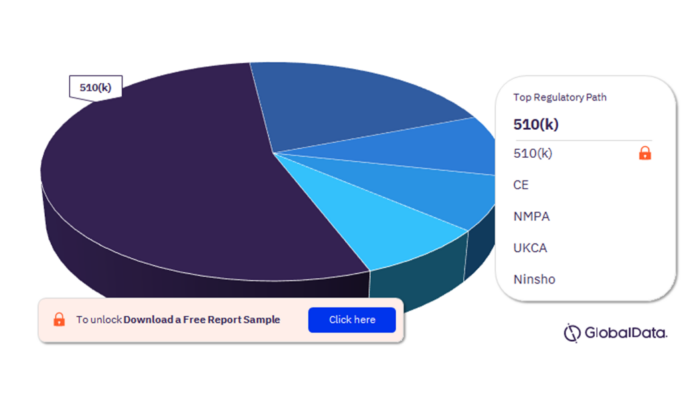
3.4 Immunochemistry Analyzers – Pipeline Products by Regulatory Path 17
3.5 Immunochemistry Analyzers – Pipeline Products by Estimated Approval Date 18
4 Immunochemistry Analyzers – Pipeline Products under Development by Companies 19
4.1 Immunochemistry Analyzers Companies – Pipeline Products by Stage of Development 19
4.2 Immunochemistry Analyzers – Pipeline Products by Stage of Development 21
5 Immunochemistry Analyzers Companies and Product Overview 23
5.1 Astek Diagnostics Company Overview 23
5.1.1 Astek Diagnostics Pipeline Products & Ongoing Clinical Trials Overview 23
5.2 Atonomics A/S (Inactive) Company Overview 24
5.2.1 Atonomics A/S (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 24
5.3 Beckman Coulter Inc Company Overview 25
5.3.1 Beckman Coulter Inc Pipeline Products & Ongoing Clinical Trials Overview 25
5.4 Biosan Medical Ltd Company Overview 26
5.4.1 Biosan Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 26
5.5 BioSIMS Company Overview 27
5.5.1 BioSIMS Pipeline Products & Ongoing Clinical Trials Overview 27
5.6 Boditech Med Inc Company Overview 28
5.6.1 Boditech Med Inc Pipeline Products & Ongoing Clinical Trials Overview 28
5.7 Boston University Company Overview 32
5.7.1 Boston University Pipeline Products & Ongoing Clinical Trials Overview 32
5.8 BreviTest Technologies LLC Company Overview 33
5.8.1 BreviTest Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 33
5.9 China Medical Technologies Inc (Inactive) Company Overview 34
5.9.1 China Medical Technologies Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 34
5.10 ChipCare Corp Company Overview 35
5.10.1 ChipCare Corp Pipeline Products & Ongoing Clinical Trials Overview 35
5.11 Clearbridge BioLoc Pte Ltd Company Overview 36
5.11.1 Clearbridge BioLoc Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 36
5.12 Corgenix Medical Corp Company Overview 37
5.12.1 Corgenix Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 37
5.13 DiaSorin SpA Company Overview 38
5.13.1 DiaSorin SpA Pipeline Products & Ongoing Clinical Trials Overview 38
5.14 Drew Scientific Co Ltd Company Overview 39
5.14.1 Drew Scientific Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 39
5.15 Dyamed Biotech Pte Ltd Company Overview 40
5.15.1 Dyamed Biotech Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 40
5.16 Enigma Diagnostics Ltd (Inactive) Company Overview 41
5.16.1 Enigma Diagnostics Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 41
5.17 Euroimmun AG Company Overview 42
5.17.1 Euroimmun AG Pipeline Products & Ongoing Clinical Trials Overview 42
5.18 Firalis SAS Company Overview 44
5.18.1 Firalis SAS Pipeline Products & Ongoing Clinical Trials Overview 44
5.19 GenapSys Inc Company Overview 45
5.19.1 GenapSys Inc Pipeline Products & Ongoing Clinical Trials Overview 45
5.20 Genevo Biosystems Pte Ltd Company Overview 48
5.20.1 Genevo Biosystems Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 48
5.21 Great Basin Scientific Inc. Company Overview 49
5.21.1 Great Basin Scientific Inc. Pipeline Products & Ongoing Clinical Trials Overview 49
5.22 HeatFlow Technologies Inc Company Overview 50
5.22.1 HeatFlow Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 50
5.23 Hokkaido University Company Overview 51
5.23.1 Hokkaido University Pipeline Products & Ongoing Clinical Trials Overview 51
5.24 Huakang Biomedical Holdings Co Ltd Company Overview 52
5.24.1 Huakang Biomedical Holdings Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 52
5.25 Indian Institute of Technology Madras Company Overview 53
5.25.1 Indian Institute of Technology Madras Pipeline Products & Ongoing Clinical Trials Overview 53
5.26 Johnson & Johnson Company Overview 54
5.26.1 Johnson & Johnson Pipeline Products & Ongoing Clinical Trials Overview 54
5.27 LabNow Inc (Inactive) Company Overview 55
5.27.1 LabNow Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 55
5.28 Lucerna Inc Company Overview 56
5.28.1 Lucerna Inc Pipeline Products & Ongoing Clinical Trials Overview 56
5.29 Magellan Diagnostics Inc Company Overview 57
5.29.1 Magellan Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 57
5.30 MedNostic Technologies, LLC Company Overview 58
5.30.1 MedNostic Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview 58
5.31 Merck Millipore Corp Company Overview 59
5.31.1 Merck Millipore Corp Pipeline Products & Ongoing Clinical Trials Overview 59
5.32 Meridian Bioscience Inc Company Overview 60
5.32.1 Meridian Bioscience Inc Pipeline Products & Ongoing Clinical Trials Overview 60
5.33 NanoLab Inc Company Overview 61
5.33.1 NanoLab Inc Pipeline Products & Ongoing Clinical Trials Overview 61
5.34 Ortho Clinical Diagnostics Holdings Plc Company Overview 63
5.34.1 Ortho Clinical Diagnostics Holdings Plc Pipeline Products & Ongoing Clinical Trials Overview 63
5.35 PerkinElmer Inc Company Overview 64
5.35.1 PerkinElmer Inc Pipeline Products & Ongoing Clinical Trials Overview 64
5.36 Protomex Life Sciences Company Overview 65
5.36.1 Protomex Life Sciences Pipeline Products & Ongoing Clinical Trials Overview 65
5.37 Randox Laboratories Ltd Company Overview 66
5.37.1 Randox Laboratories Ltd Pipeline Products & Ongoing Clinical Trials Overview 66
5.38 Rapid Diagnostek, Inc. Company Overview 67
5.38.1 Rapid Diagnostek, Inc. Pipeline Products & Ongoing Clinical Trials Overview 67
5.39 Siloam Biosciences, Inc. Company Overview 68
5.39.1 Siloam Biosciences, Inc. Pipeline Products & Ongoing Clinical Trials Overview 68
5.40 Spectradyne LLC Company Overview 69
5.40.1 Spectradyne LLC Pipeline Products & Ongoing Clinical Trials Overview 69
5.41 Tecan Group Ltd Company Overview 70
5.41.1 Tecan Group Ltd Pipeline Products & Ongoing Clinical Trials Overview 70
5.42 The Palo Alto Research Center Company Overview 71
5.42.1 The Palo Alto Research Center Pipeline Products & Ongoing Clinical Trials Overview 71
5.43 Tyncbest Biotechnology Company Overview 72
5.43.1 Tyncbest Biotechnology Pipeline Products & Ongoing Clinical Trials Overview 72
5.44 University of Illinois at Urbana-Champaign Company Overview 73
5.44.1 University of Illinois at Urbana-Champaign Pipeline Products & Ongoing Clinical Trials Overview 73
5.45 University of Michigan Pediatric Device Consortium Company Overview 74
5.45.1 University of Michigan Pediatric Device Consortium Pipeline Products & Ongoing Clinical Trials Overview 74
5.46 University of South Florida Company Overview 75
5.46.1 University of South Florida Pipeline Products & Ongoing Clinical Trials Overview 75
5.47 University of Southern California Company Overview 76
5.47.1 University of Southern California Pipeline Products & Ongoing Clinical Trials Overview 76
5.48 Virogenomics BioDevelopment Inc Company Overview 77
5.48.1 Virogenomics BioDevelopment Inc Pipeline Products & Ongoing Clinical Trials Overview 77
5.49 Xanthostat Diagnostics Inc Company Overview 78
5.49.1 Xanthostat Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 78
5.50 Yunnan Energy International Co Ltd Company Overview 79
5.50.1 Yunnan Energy International Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 79
6 Immunochemistry Analyzers- Recent Developments 80
6.1 May 13, 2022: Siemens to exit Russia over invasion of Ukraine; takes €600m hit 80
6.2 May 12, 2022: Siemens Strong operational performance and growth ended March 31, 2022 80
6.3 May 12, 2022: Johnson & Johnson to Participate in Goldman Sachs 43rd Annual Global Healthcare Conference 82
6.4 May 12, 2022: Siemens Announces Q2 FY 2022 results 82
6.5 May 11, 2022: Johnson & Johnson Appoints Thibaut Mongon as CEO Designate of Planned New Consumer Health Company 83
6.6 May 11, 2022: IsoPlexis Reports First Quarter 2022 Financial Results 83
6.7 May 11, 2022: Babson, BD expand strategic partnership to advance diagnostic blood collection in new care settings 84
6.8 May 10, 2022: Johnson & Johnson MedTech Adds New CFO to the Mix 85
6.9 May 04, 2022: Ortho Clinical Diagnostics Reports First Quarter 2022 Results 86
6.10 May 02, 2022: Johnson & Johnson to Participate in the Bernstein 38th Annual Strategic Decisions Conference 87
6.11 Apr 29, 2022: BD to Present at the BofA Securities 2022 Healthcare Conference 87
6.12 Apr 28, 2022: Novacyt Full year 2021 results and update on growth strategy 87
6.13 Apr 25, 2022: EKF Diagnostics Holdings announces Posting of Annual Report & Accounts &Notice of AGM 89
6.14 Apr 20, 2022: Johnson & Johnson to Participate in the UBS Global Healthcare Conference 90
6.15 Apr 20, 2022: Ortho Clinical Diagnostics to Report First Quarter 2022 Results on May 4, 2022 90
6.16 Apr 19, 2022: Johnson & Johnson Reports Q1 2022 Results 90
6.17 Apr 12, 2022: Tecan announce Annual General Meeting 2022 91
6.18 Apr 12, 2022: BIO-TECHNE and Cygnus Technologies, part of Maravai LifeSciences, introduce new simple plex HEK 293 HCP 3G immunoassay 92
6.19 Apr 11, 2022: Thermo Fisher Scientific to Host Investor Day 93
6.20 Apr 11, 2022: IsoPlexis Reports Preliminary First Quarter 2022 Revenue, Streamlines Operating Structure to Support Sustainable Growth 93
6.21 Apr 07, 2022: Quidel Announces Preliminary Revenue for First Quarter 2022 94
6.22 Apr 07, 2022: Johnson & Johnson to Participate in the BofA Securities 2022 Healthcare Conference 95
6.23 Apr 06, 2022: BioPorto Notice Convening the Annual General Meeting 95
6.24 Mar 15, 2022: Tecan closes transformative year with substantial growth in sales and net profit 95
6.25 Mar 15, 2022: Johnson & Johnson to Host Investor Conference Call on First-Quarter Results 99
6.26 Mar 14, 2022: Qiagen Announces 20-f Annual Report Filing for 2021 Results 100
6.27 Mar 09, 2022: Bioasis to Attend and Present at Upcoming Industry Conference 100
6.28 Mar 01, 2022: Cardiff Oncology to Participate in Cowen’s 42nd Annual Healthcare Conference 100
6.29 Feb 25, 2022: Bio-Rad presents growth strategy and acceleration of financial targets at investor day 101
6.30 Feb 24, 2022: BD to Present at the Cowen 42nd Annual Health Care Conference 101
6.31 Feb 23, 2022: embecta to Host Virtual Investor Event Ahead of Planned Spinoff from BD 102
6.32 Feb 23, 2022: Bd Appoints Shana Neal as Chief People Officer 102
6.33 Feb 16, 2022: Ortho Clinical Diagnostics Reports Fourth Quarter and Fiscal Year 2021 Results 102
6.34 Feb 15, 2022: Johnson & Johnson Names Darius Adamczyk, Chairman and Chief Executive Officer of Honeywell, to its Board of Directors 104
6.35 Feb 14, 2022: Bio-Rad Laboratories to Post Q1 2022 Earnings of $2.80 Per Share, Jefferies Financial Group Forecasts 104
6.36 Feb 14, 2022: Horiba announces Summary of Consolidated Financial Statements for the Year Ended December 31, 2021 104
6.37 Feb 11, 2022: KAIST develops rapid bacterial detection method using deep learning 105
6.38 Feb 11, 2022: Span Divergent Un-Audited Financial Results for the Quarter Ended 31st December 2021 106
6.39 Feb 09, 2022: Johnson & Johnson to Participate in the Raymond James 43rd Annual Institutional Investors Conference 106
6.40 Feb 07, 2022: Johnson & Johnson to Participate in the Cowen 42nd Annual Health Care Virtual Conference 106
6.41 Feb 02, 2022: Bio-Rad to Host Investor Day on February 25 107
6.42 Jan 31, 2022: EKF Diagnostics Holdings Announces Non-executive Directorate Change 107
6.43 Jan 27, 2022: Johnson & Johnson to Participate in Citi’s 2022 Virtual Healthcare Conference 108
6.44 Jan 25, 2022: Johnson & Johnson Reports Q4 and Full-Year 2021 Results 108
6.45 Jan 11, 2022: Cardiff Oncology Appoints Tod Smeal, Ph.D., as Chief Scientific Officer and Charles Monahan, RPh, as Senior Vice President, Regulatory Affairs 109
6.46 Jan 11, 2022: SDI Group Announces Board Change 110
6.47 Jan 07, 2022: Quidel Announces Preliminary Revenue for Fourth Quarter 2021 110
6.48 Jan 06, 2022: BD Announces Change to Virtual 2022 Annual Meeting of Shareholders 111
6.49 Jan 05, 2022: BD to announce financial results for its first quarter of fiscal year 2022 112
6.50 Jan 04, 2022: Halo Collective appoints Sky Pinnick as chief marketing officer 112
6.51 Jan 03, 2022: Ortho Clinical Diagnostics to Participate in the 40th Annual JP Morgan Healthcare Conference 113
6.52 Jan 02, 2022: Ortho Clinical Diagnostics Announces Preliminary Financial Results for Fourth Quarter and Fiscal Year 2021 113
6.53 Dec 21, 2021: BD Announces Board of Directors for embecta, the Planned Spinoff of its Diabetes Care Business 114
6.54 Dec 10, 2021: Johnson & Johnson to Host Investor Conference Call on Fourth-Quarter Results 116
6.55 Dec 08, 2021: Johnson & Johnson to Participate in the 40th Annual J.P. Morgan Healthcare Conference 117
6.56 Dec 07, 2021: SDI Group Announces Interim results for the six months ended 31 October 2021 117
6.57 Dec 07, 2021: SDI Group – Interim Results for the six months ended 31 October 2021 118
6.58 Nov 29, 2021: Bioasis Announces Annual General Meeting Details 119
6.59 Nov 26, 2021: Mitsubishi Chemical Holdings Notice of Changes Regarding the Corporate Executive Officer and Chief Financial Officer 120
6.60 Nov 23, 2021: EKF Diagnostics Holdings Appoints Marc Davies as Chief Financial Officer 120
6.61 Nov 23, 2021: Biolidics Announces Appointment of Independent Director 121
6.62 Nov 22, 2021: RD-Biotech expands antibody and immunoassay services with xMAP Technology 121
6.63 Nov 15, 2021: BioPorto Announces Extraordinary General Meeting and New Board Member 122
6.64 Nov 12, 2021: Psychemedics Reports Stronger Revenue, Operating Profit and Earnings Growth in Third Quarter 2021 122
6.65 Nov 11, 2021: Horiba Announces Summary of Consolidated Financial Statements for the Nine Months Ended September 30, 2021 124
6.66 Nov 10, 2021: Sysmex announces Summary of Consolidated Financial Results for the First Six Months of the Fiscal Year Ending March 31, 2022 125
6.67 Nov 10, 2021: Sysmex Announcement Regarding Differences between Actual and Forecast Figures for the Six Months Ended September 30, 2021, and Revision of Full-Year Financial Forecasts 127
6.68 Nov 04, 2021: Quidel Reports Third Quarter 2021 Financial Results 128
6.69 Nov 04, 2021: Cardiff Oncology Reports Third Quarter 2021 Results and Provides Business Updates 130
6.70 Oct 29, 2021: Halo Collective announces “Budega” as name for retail store brand, appoints new retail senior vice president 133
6.71 Oct 26, 2021: BD to Host Investor Day on November 12 133
6.72 Oct 20, 2021: BioPorto appoints new Chief Executive Officer and announces changes to the Board of Directors 134
6.73 Oct 20, 2021: BioPorto appoints new Chief Financial Officer 134
6.74 Oct 12, 2021: Dr. Paul Stoffels, Vice Chairman of the Executive Committee and Chief Scientific Officer of Johnson & Johnson To Retire, Effective December 31, 2021 135
6.75 Oct 12, 2021: Johnson & Johnson Announces Jessica Moore as Vice President of Investor Relations 136
6.76 Oct 12, 2021: Ortho Clinical Diagnostics to Report Third Quarter 2021 Results on November 3, 2021 137
6.77 Oct 11, 2021: Johnson & Johnson to Participate in the Credit Suisse 30th Annual Healthcare Conference 137
6.78 Oct 07, 2021: Quidel Announces Preliminary Revenue for Fiscal Third Quarter 2021 137
6.79 Oct 01, 2021: Mitsubishi Chemical Holdings announces Personnel Personnel Changes 138
6.80 Sep 30, 2021: Bioasis to Attend Upcoming Industry Conferences 138
6.81 Sep 29, 2021: Novacyt Announces Annual General Meeting 139
6.82 Sep 29, 2021: Novacyt Notice of AGM 139
6.83 Sep 27, 2021: Novacyt reports 1H results 140
6.84 Sep 27, 2021: Novacyt Announces unaudited results for the half year ended 30 June 2021 140
6.85 Sep 16, 2021: Lynette Jackson appointed as new head of communications at Siemens 141
6.86 Sep 16, 2021: Johnson & Johnson to Host Investor Conference Call on Third-Quarter Results 142
6.87 Sep 14, 2021: EKF Diagnostics Holdings Announces unaudited interim results for the six months ended 30 June 2021 142
6.88 Sep 13, 2021: SQI Diagnostics Appoints Andrew Morris as Chief Executive Officer, and Grants Options 143
6.89 Sep 03, 2021: Ortho Clinical Diagnostics to Participate in September Investor Conferences 144
6.90 Sep 02, 2021: Hologic to Webcast Presentations at Upcoming Virtual Investor Conferences 144
6.91 Sep 01, 2021: BioMerieux announce First-Half 2021 Results 145
6.92 Sep 01, 2021: Biolidics Announces Re-designation of Interim Chief Executive Officer as Chief Scientific Officer 145
6.93 Sep 01, 2021: Biolidics Appointment of Director of Corporate Finance and Corporate Development 145
6.94 Sep 01, 2021: Biolidics Announces Appointment of Executive Director and Chief Executive Officer 145
6.95 Aug 23, 2021: SDI Group Posting of Annual Report and Notice of AGM 146
6.96 Aug 20, 2021: Novacyt Notice of AGM 146
6.97 Aug 19, 2021: Alex Gorsky to Serve as Executive Chairman and Transition Role of Chief Executive Officer of Johnson & Johnson to Joaquin Duato, Effective January 3, 2022 146
6.98 Aug 18, 2021: BioPorto Announces Q2 2021 Report 148
6.99 Aug 16, 2021: Halo Collective releases Q2 2021 results 149
6.100 Aug 13, 2021: Biolidics’ Revenue Dipped to S$0.64 Million in the First Half of 2021 151
6.101 Aug 13, 2021: A multiplex TaqMan qPCR assay for detection and quantification of clade 1 and clade 2 isolates of Pseudoperonospora cubensis and Pseudoperonospora humuli 152
6.102 Aug 13, 2021: A novel multiplex qPCR method for assessing the comparative lengths of telomeres 154
6.103 Aug 12, 2021: Johnson & Johnson to Participate in the Morgan Stanley 19th Annual Global Healthcare Conference 155
6.104 Aug 11, 2021: Span Divergent announces Result For The Quarter Ended On June 30, 2021 155
6.105 Aug 10, 2021: Horiba Reports Consolidated Financial Statements for the Six Months Ended June 30, 2021 156
6.106 Aug 09, 2021: Johnson & Johnson to Participate in Barclays Global Consumer Staples Virtual Conference 157
6.107 Aug 06, 2021: Sysmex Provides Summary of Consolidated Financial Results for the First Three Months of the Fiscal Year Ending March 31, 2022 157
6.108 Aug 05, 2021: Siemens Announces Excellent Results with Raised Guidance 159
6.109 Aug 04, 2021: Ortho Clinical Diagnostics Reports Second Quarter 2021 Results 161
6.110 Aug 04, 2021: Mitsubishi Chemical Holdings announces Financial Results Consolidated Financial Results for the First Quarter of the Fiscal Year Ending March 31, 2022 163
6.111 Aug 04, 2021: Mitsubishi Chemical Holdings Announces Notice Regarding Revision to Consolidated Financial Results Forecast for the First Half of Fiscal Year Ending March 31, 2022 163
6.112 Jul 29, 2021: Bio-Rad Reports Second-Quarter 2021 Financial Results 163
6.113 Jul 29, 2021: QIAGEN Reports Full Results for Q2 and H1 2021 164
6.114 Jul 29, 2021: BD to Present at UBS Genomics
2.0 and MedTech Innovations Summit 166
6.115 Jul 29, 2021: Bioasis Announces Filing of Its First Quarter Financial Statements and MD&A 166
6.116 Jul 28, 2021: Hologic Announces Financial Results for Third Quarter of Fiscal 2021 167
6.117 Jul 27, 2021: Johnson & Johnson to Participate in the Virtual Wells Fargo Healthcare Conference 168
6.118 Jul 23, 2021: Ortho Clinical Diagnostics to Report Second Quarter 2021 Results on August 4, 2021 168
6.119 Jul 21, 2021: Johnson & Johnson Reports 2021 Second-Quarter Results 168
6.120 Jul 19, 2021: Theradiag Reports Revenue of €5.5 Million for the First Half of 2021, Up
12.5% 169
6.121 Jul 01, 2021: Bioasis Announces the Appointment of Dave Jenkins as Chief Financial Officer 171
6.122 Jun 24, 2021: Mitsubishi Chemical Holdings announces Personnel Notice Regarding Executive Personnel Changes 171
6.123 Jun 23, 2021: Halo Collective Announces Results of Annual General and Special Meeting 172
6.124 Jun 22, 2021: Novacyt Full Year 2020 Results and Update on Growth Strategy 172
6.125 Jun 22, 2021: Novacyt Announces Full year 2020 results and update on growth strategy 184
6.126 Jun 16, 2021: Mitsubishi Chemical Announces Personnel Organizational Changes and Personnel Changes 184
6.127 Jun 14, 2021: Biolidics Announces Appointment of Ian David Brown as an Independent Director 185
6.128 Jun 11, 2021: Span Divergent announces Financial Results For The Year Ended On March 31, 2021 185
7 Appendix 186
7.1 Methodology 186
7.2 About GlobalData 188
7.3 Contact Us 189
7.4 Disclaimer 189
![]()