|1 Table of Contents 3
|1.1 List of Tables 9
|1.2 List of Figures 14
2 Introduction 15
2.1 Infusion Systems Overview 15
3 Products under Development 16
3.1 Infusion Systems – Pipeline Products by Stage of Development 16
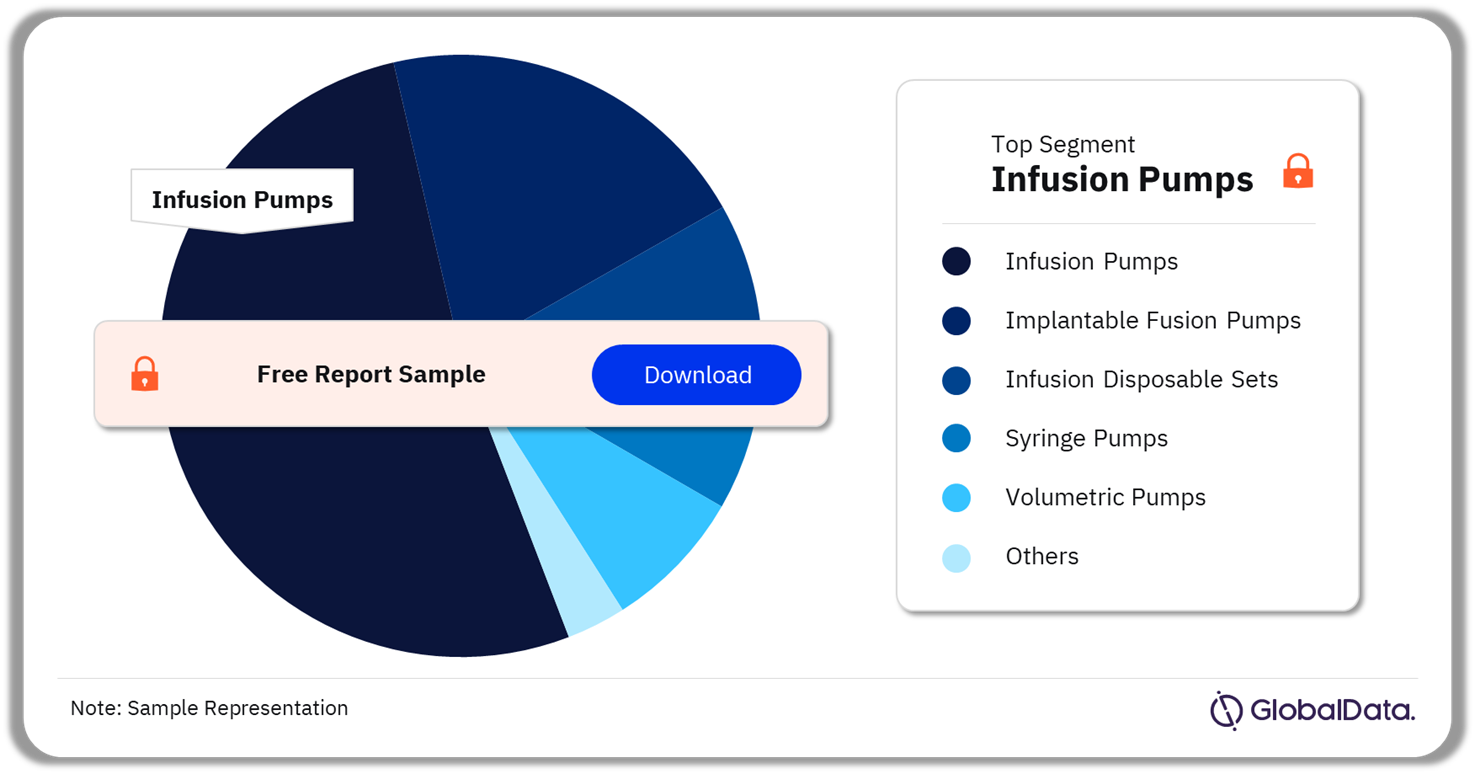
3.2 Infusion Systems – Pipeline Products by Segment 17
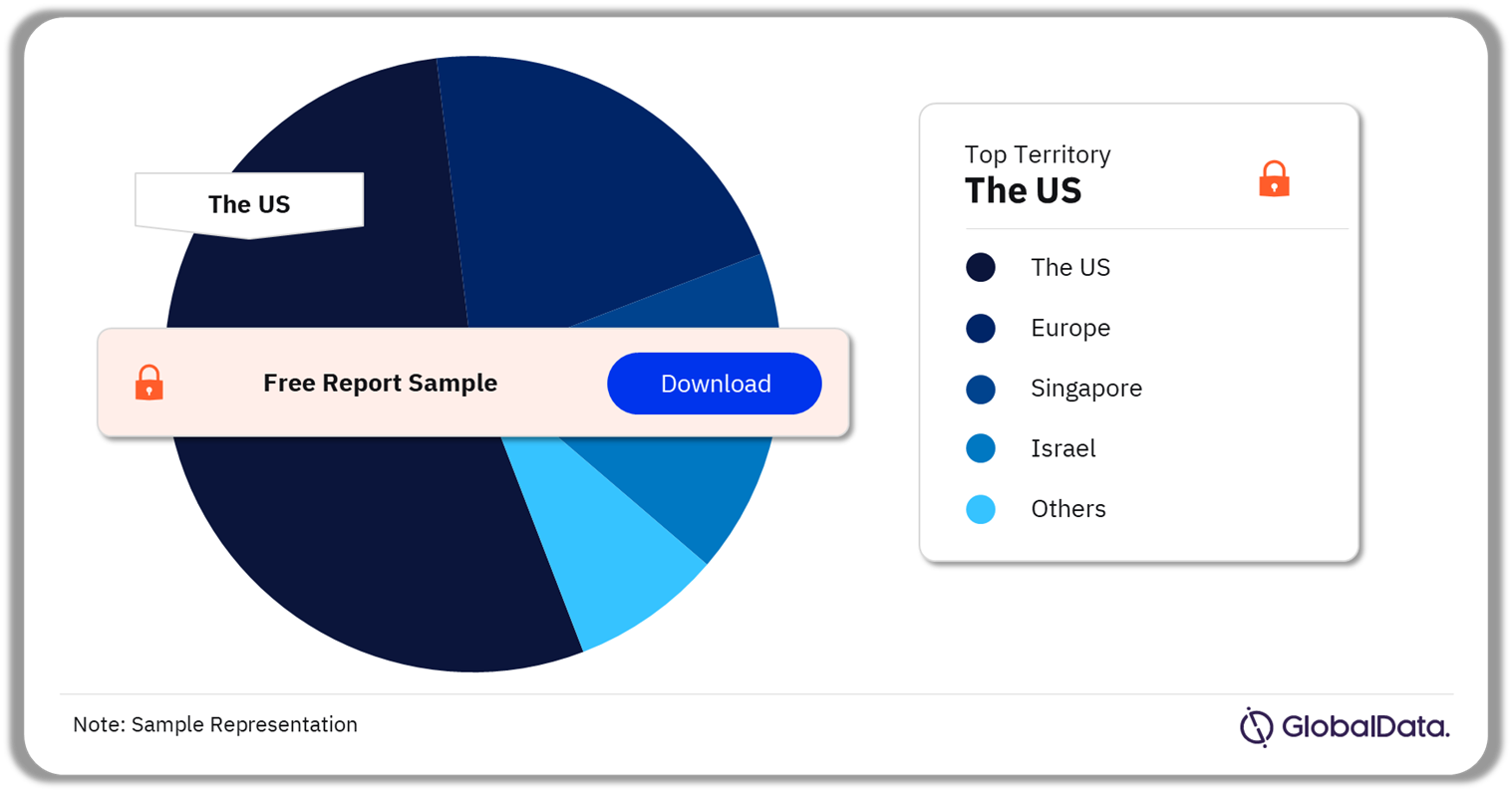
3.3 Infusion Systems – Pipeline Products by Territory 18
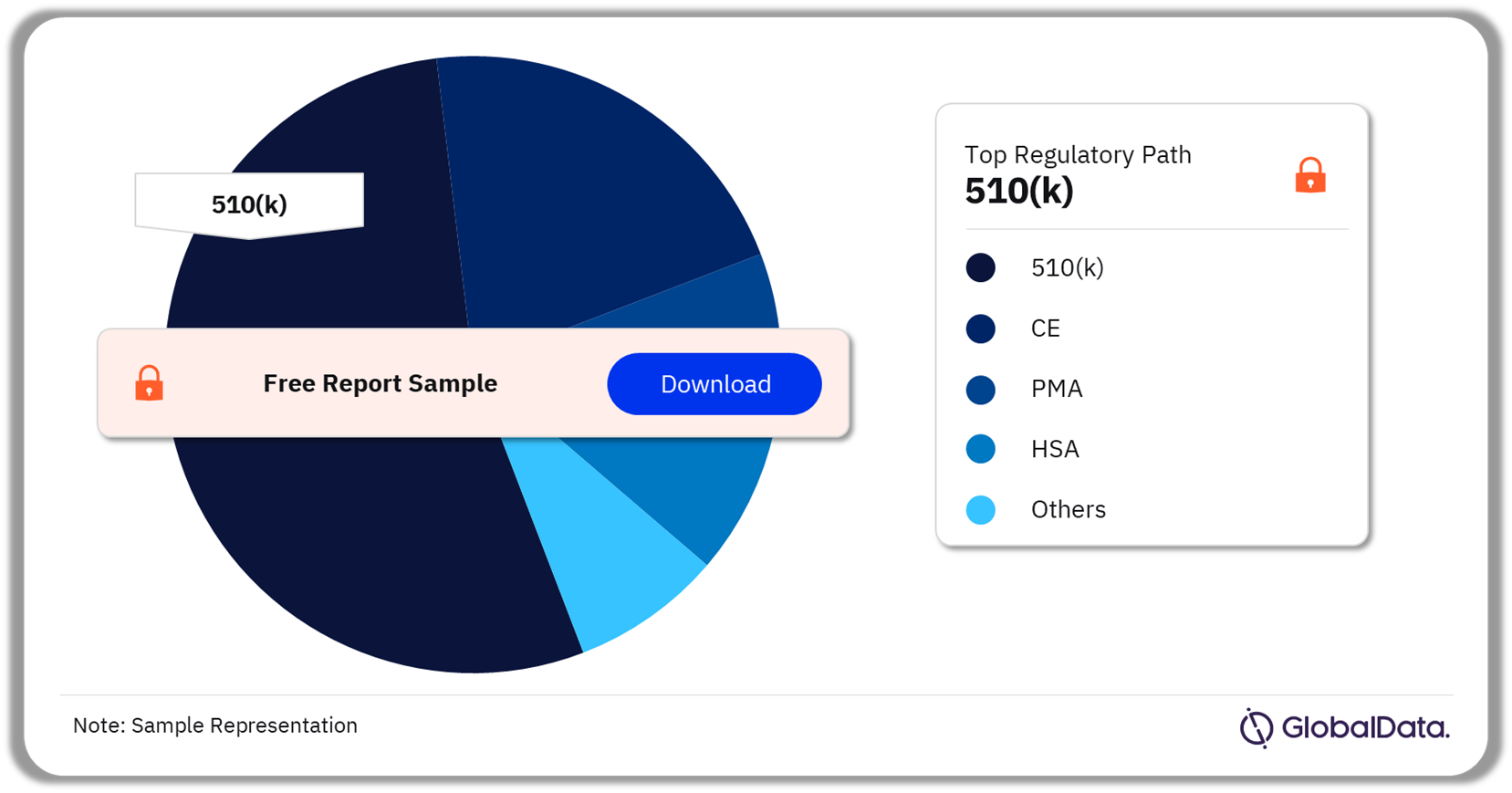
3.4 Infusion Systems – Pipeline Products by Regulatory Path 19
3.5 Infusion Systems – Pipeline Products by Estimated Approval Date 20
3.6 Infusion Systems – Ongoing Clinical Trials 21
4 Infusion Systems – Pipeline Products under Development by Companies 22
4.1 Infusion Systems Companies – Pipeline Products by Stage of Development 22
4.2 Infusion Systems – Pipeline Products by Stage of Development 25
5 Infusion Systems Companies and Product Overview 28
5.1 410 Medical Inc Company Overview 28
5.1.1 410 Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 28
5.2 AbbVie Inc Company Overview 29
5.2.1 AbbVie Inc Pipeline Products & Ongoing Clinical Trials Overview 29
5.3 Acuros GmbH Company Overview 30
5.3.1 Acuros GmbH Pipeline Products & Ongoing Clinical Trials Overview 30
5.4 Adept Medical Ltd Company Overview 31
5.4.1 Adept Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 31
5.5 Agitated Solutions LLC Company Overview 32
5.5.1 Agitated Solutions LLC Pipeline Products & Ongoing Clinical Trials Overview 32
5.6 Albireo Pharma Inc Company Overview 33
5.6.1 Albireo Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 33
5.7 Alnylam Pharmaceuticals Inc Company Overview 34
5.7.1 Alnylam Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 34
5.8 Arnali, LLC Company Overview 35
5.8.1 Arnali, LLC Pipeline Products & Ongoing Clinical Trials Overview 35
5.9 Avoset Health Ltd Company Overview 36
5.9.1 Avoset Health Ltd Pipeline Products & Ongoing Clinical Trials Overview 36
5.10 Baxter Healthcare Corp Company Overview 37
5.10.1 Baxter Healthcare Corp Pipeline Products & Ongoing Clinical Trials Overview 37
5.11 Baxter International Inc Company Overview 40
5.11.1 Baxter International Inc Pipeline Products & Ongoing Clinical Trials Overview 40
5.12 Becton Dickinson and Co Company Overview 42
5.12.1 Becton Dickinson and Co Pipeline Products & Ongoing Clinical Trials Overview 42
5.13 BioQ Pharma Inc Company Overview 44
5.13.1 BioQ Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 44
5.14 ClearLine MD Company Overview 46
5.14.1 ClearLine MD Pipeline Products & Ongoing Clinical Trials Overview 46
5.15 Cognos Therapeutics Inc Company Overview 47
5.15.1 Cognos Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 47
5.16 Debiotech SA Company Overview 48
5.16.1 Debiotech SA Pipeline Products & Ongoing Clinical Trials Overview 48
5.17 Eksigent Technologies, LLC Company Overview 50
5.17.1 Eksigent Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview 50
5.18 Eli Lilly and Co Company Overview 52
5.18.1 Eli Lilly and Co Pipeline Products & Ongoing Clinical Trials Overview 52
5.19 EryDel SPA Company Overview 53
5.19.1 EryDel SPA Pipeline Products & Ongoing Clinical Trials Overview 53
5.20 Flowonix Medical Inc Company Overview 56
5.20.1 Flowonix Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 56
5.21 Fluid Synchrony LLC Company Overview 58
5.21.1 Fluid Synchrony LLC Pipeline Products & Ongoing Clinical Trials Overview 58
5.22 Fluonic, Inc. (Inactive) Company Overview 59
5.22.1 Fluonic, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 59
5.23 Fresenius Medical Care AG & Co KGaA Company Overview 60
5.23.1 Fresenius Medical Care AG & Co KGaA Pipeline Products & Ongoing Clinical Trials Overview 60
5.24 HAI Solutions Inc Company Overview 61
5.24.1 HAI Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 61
5.25 Hospira Inc Company Overview 62
5.25.1 Hospira Inc Pipeline Products & Ongoing Clinical Trials Overview 62
5.26 ICU Medical Inc Company Overview 64
5.26.1 ICU Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 64
5.27 Infusense Corp Company Overview 66
5.27.1 Infusense Corp Pipeline Products & Ongoing Clinical Trials Overview 66
5.28 Innofuse BV Company Overview 67
5.28.1 Innofuse BV Pipeline Products & Ongoing Clinical Trials Overview 67
5.29 Innovative Health Sciences LLC Company Overview 68
5.29.1 Innovative Health Sciences LLC Pipeline Products & Ongoing Clinical Trials Overview 68
5.30 Innovfusion Pte. Ltd. Company Overview 69
5.30.1 Innovfusion Pte. Ltd. Pipeline Products & Ongoing Clinical Trials Overview 69
5.31 Intarcia Therapeutics Inc Company Overview 72
5.31.1 Intarcia Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 72
5.32 IRadimed Corp Company Overview 73
5.32.1 IRadimed Corp Pipeline Products & Ongoing Clinical Trials Overview 73
5.33 KORU Medical Systems Inc Company Overview 74
5.33.1 KORU Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 74
5.34 LifeMedix, LLC Company Overview 75
5.34.1 LifeMedix, LLC Pipeline Products & Ongoing Clinical Trials Overview 75
5.35 Lynntech Inc Company Overview 76
5.35.1 Lynntech Inc Pipeline Products & Ongoing Clinical Trials Overview 76
5.36 Massachusetts Institute of Technology Company Overview 77
5.36.1 Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 77
5.37 Medical Device Creations Ltd. Company Overview 80
5.37.1 Medical Device Creations Ltd. Pipeline Products & Ongoing Clinical Trials Overview 80
5.38 Medovate Ltd Company Overview 81
5.38.1 Medovate Ltd Pipeline Products & Ongoing Clinical Trials Overview 81
5.39 MonuMedical LLC Company Overview 82
5.39.1 MonuMedical LLC Pipeline Products & Ongoing Clinical Trials Overview 82
5.40 Neurochase Ltd Company Overview 83
5.40.1 Neurochase Ltd Pipeline Products & Ongoing Clinical Trials Overview 83
5.41 NexGen Medical Systems Inc Company Overview 84
5.41.1 NexGen Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 84
5.42 Palyon Medical Corp (Inactive) Company Overview 85
5.42.1 Palyon Medical Corp (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 85
5.43 Pavmed Inc Company Overview 86
5.43.1 Pavmed Inc Pipeline Products & Ongoing Clinical Trials Overview 86
5.44 PRO-IV Medical Ltd Company Overview 91
5.44.1 PRO-IV Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 91
5.45 Ratio Inc Company Overview 92
5.45.1 Ratio Inc Pipeline Products & Ongoing Clinical Trials Overview 92
5.46 Rice University Company Overview 93
5.46.1 Rice University Pipeline Products & Ongoing Clinical Trials Overview 93
5.47 Sensile Medical AG Company Overview 94
5.47.1 Sensile Medical AG Pipeline Products & Ongoing Clinical Trials Overview 94
5.48 Shenox Pharmaceuticals LLC Company Overview 95
5.48.1 Shenox Pharmaceuticals LLC Pipeline Products & Ongoing Clinical Trials Overview 95
5.49 Shift Labs Inc Company Overview 96
5.49.1 Shift Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 96
5.50 SQ Innovation AG Company Overview 97
5.50.1 SQ Innovation AG Pipeline Products & Ongoing Clinical Trials Overview 97
5.51 Sree Chitra Tirunal Institute for Medical Sciences & Technology Company Overview 100
5.51.1 Sree Chitra Tirunal Institute for Medical Sciences & Technology Pipeline Products & Ongoing Clinical Trials Overview 100
5.52 Starton Therapeutics Inc Company Overview 101
5.52.1 Starton Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 101
5.53 SteadyMed Therapeutics Inc Company Overview 102
5.53.1 SteadyMed Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 102
5.54 StnDrd Infusion Corporation Company Overview 104
5.54.1 StnDrd Infusion Corporation Pipeline Products & Ongoing Clinical Trials Overview 104
5.55 Supernus Pharmaceuticals Inc Company Overview 105
5.55.1 Supernus Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 105
5.56 Terumo Corp Company Overview 106
5.56.1 Terumo Corp Pipeline Products & Ongoing Clinical Trials Overview 106
5.57 ToucheMedical Ltd. Company Overview 107
5.57.1 ToucheMedical Ltd. Pipeline Products & Ongoing Clinical Trials Overview 107
5.58 TriSalus Life Sciences Inc Company Overview 108
5.58.1 TriSalus Life Sciences Inc Pipeline Products & Ongoing Clinical Trials Overview 108
5.59 Turnpoint Medical Devices Inc Company Overview 110
5.59.1 Turnpoint Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 110
5.60 U-Needle BV Company Overview 111
5.60.1 U-Needle BV Pipeline Products & Ongoing Clinical Trials Overview 111
5.61 Unilife Corporation Company Overview 113
5.61.1 Unilife Corporation Pipeline Products & Ongoing Clinical Trials Overview 113
5.62 United Therapeutics Corp Company Overview 114
5.62.1 United Therapeutics Corp Pipeline Products & Ongoing Clinical Trials Overview 114
5.63 University of Minnesota Company Overview 116
5.63.1 University of Minnesota Pipeline Products & Ongoing Clinical Trials Overview 116
5.64 University of Southern California Company Overview 117
5.64.1 University of Southern California Pipeline Products & Ongoing Clinical Trials Overview 117
5.65 Zealand Pharma AS Company Overview 118
5.65.1 Zealand Pharma AS Pipeline Products & Ongoing Clinical Trials Overview 118
6 Infusion Systems- Recent Developments 119
6.1 Nov 14, 2023: KORU Medical Systems Receives Additional 510(K) Clearance For FREEDOM60 119
6.2 Nov 14, 2023: KORU Medical Systems Receives Additional 510(K) Clearance For HIgH-Flo Super26 Subcutaneous Safety Needle Set 119
6.3 Nov 14, 2023: KORU Medical Systems Receives Additional 510(K) Clearance For HIgH-Flo Subcutaneous Safety Needle Set 119
6.4 Nov 10, 2023: Microport Appointment of Director 120
6.5 Nov 09, 2023: 3M Announces Leadership Changes 120
6.6 Nov 08, 2023: Koru Medical Systems Announces Development Agreement for Next-Generation Subcutaneous Immunoglobulin Infusion System 121
6.7 Nov 08, 2023: KORU Medical Systems, Appoints Ken Miller as Chief Commercial Officer 121
6.8 Nov 08, 2023: KORU Medical Systems Announces 2023 Third Quarter Financial Results and Updates Full Year 2023 Outlook 122
6.9 Nov 07, 2023: Koru Medical Systems Receives FDA 510(K) Clearance for FREEDOM60 Infusion System With Hizentra 50 ML Prefilled Syringes 123
6.10 Nov 05, 2023: Teleflex Reports Third Quarter Financial Results, Full Year 2023 Outlook 124
6.11 Nov 03, 2023: Cardinal Health Reports First Quarter Fiscal 2024 Results and Raises Fiscal 2024 Outlook 124
6.12 Oct 26, 2023: Lilly to Participate in UBS Biopharma Conference 2023 125
6.13 Oct 24, 2023: 3M Reports Third Quarter 2023 Results; Company Increases Full Year Adjusted Earnings and Cash Flow Expectations 125
6.14 Oct 20, 2023: Lilly Confirms Date and Conference Call for Third-Quarter 2023 Financial Results Announcement 126
6.15 Oct 18, 2023: Edwards Lifesciences to Host Earnings Conference Call on October 25, 2023 126
6.16 Oct 09, 2023: Supernus Resubmits NDA for SPN-830 Apomorphine Infusion Device 127
6.17 Oct 05, 2023: KORU Medical Systems to Present Two Abstracts at the Immunoglobulin National Society 2023 National Conference 127
6.18 Oct 04, 2023: Lilly Announces Leadership Transitions 128
6.19 Oct 04, 2023: KORU Medical Systems Announces Appointment of Edward Wholihan to Its Board of Directors 129
6.20 Oct 04, 2023: Medtronic Names Paolo Di Vincenzo President of the Neuromodulation Business 129
6.21 Oct 03, 2023: Baxter Appoints Joel Grade Chief Financial Officer 130
6.22 Oct 02, 2023: Fresenius Kabi Selected for Second Consecutive Year to Exhibit the Ivenix Infusion System at Vizient Innovative Technology Exchange 130
6.23 Sep 21, 2023: Microport Scientific Corp. Announces 2023 Interim Report 131
6.24 Sep 19, 2023: Baxter to Host Third-Quarter 2023 Financial Results Conference Call for Investors 133
6.25 Sep 14, 2023: ICU Medical Receives 510(k) Clearance for Plum Duo Infusion System 133
6.26 Sep 14, 2023: Virginia Oncology Associates Chooses the Ivenix Infusion System from Fresenius Kabi 133
6.27 Aug 28, 2023: Edwards Lifesciences to Present at the 2023 Wells Fargo Healthcare Conference 134
6.28 Aug 26, 2023: Medtronic Q2 2024 Earnings Estimates Increased by William Blair 134
6.29 Aug 23, 2023: 3M Appoints Carrie Cox as Chairman of the Board of Directors of the Independent Health Care Company 134
6.30 Aug 22, 2023: Bryan Hanson Named CEO of 3M’s Health Care Business Group 134
6.31 Aug 15, 2023: Convatec Group Appoints Robyn Butler-Mason as Company Secretary 136
6.32 Aug 15, 2023: Cardinal Health Reports Fourth Quarter and Full Year Results for Fiscal Year 2023 at High End of Investor Day Guidance 136
6.33 Aug 08, 2023: Medtronic to Announce Financial Results for its First Quarter of Fiscal Year 2024 136
6.34 Aug 03, 2023: Teleflex Reports Second Quarter Financial Results and Full Year 2023 Outlook 137
6.35 Aug 02, 2023: ConvaTec Group Announces Interim Results for the six months ended 30 June 2023 140
6.36 Aug 01, 2023: Baxter recalls nearly 23,000 infusion pumps in Class I recall 141
6.37 Aug 01, 2023: Baxter Healthcare Corporation Recalls SIGMA Spectrum Infusion Pumps with Master Drug Library and Spectrum IQ Infusion Systems with Dose IQ Safety Software for Repeat Upstream Occlusion False Alarms 141
6.38 Jul 27, 2023: Bexson Biomedical Wearable Ketamine Device to be Tested During U.S. Military Training Exercise in August 142
6.39 Jul 24, 2023: BD CEO Tom Polen Addresses FDA 510(k) Clearance of BD Alaris Infusion System 143
6.40 Jul 17, 2023: Ablative Solutions Receives 510(k) Clearance for Peregrine System Infusion Catheter 3-7 mm 143
6.41 Jul 11, 2023: Microport Scientific Provides Consolidated Earnings Guidance for the Six Months Ended June 30, 2023 144
6.42 Jul 10, 2023: Koru Medical submits PMA for infusion system 144
6.43 Jul 10, 2023: Fresenius Medical Care appoints Martin Fischer as Chief Financial Officer 144
6.44 Jul 07, 2023: Terumo Receives Additional 510(k) Clearance for SURFLO Winged Infusion Set With Needle Protection 145
6.45 Jul 05, 2023: Gilero Receives 510(k) Clearance for UTC 3mL Medication Cartridge 145
6.46 Jun 29, 2023: Metrodora Institute, a Leader in Treating Complex Conditions, Chooses the Ivenix Infusion System From Fresenius Kabi 146
6.47 Jun 13, 2023: Medimop Medical Receives 510(k) Clearance for Vented Vial Adapter Transfer Device 146
6.48 Jun 07, 2023: Teleflex receives FDA approval for MR Conditional labelling of EZ-IO Needle 146
6.49 Jun 01, 2023: B. Braun launches DoseTrac Enterprise Infusion Management Software 147
6.50 May 23, 2023: Fresenius Kabi awarded breakthrough technology agreement with Premier, Inc. for the Ivenix Infusion System 147
6.51 May 23, 2023: Fresenius Kabi awarded technology breakthrough agreement with Premier for the Ivenix infusion system 148
6.52 May 16, 2023: TriSalus Life Sciences receives 510(k) clearance for TriNav LV Infusion System 148
6.53 May 16, 2023: PAVmed provides business update and Q1 financial results 149
6.54 May 15, 2023: Terumo announces consolidated financial results for the fiscal year ended March 31, 2023 150
6.55 May 11, 2023: Medtronic to announce financial results for its fourth quarter and full fiscal year 2023 150
6.56 May 09, 2023: 3M annual meeting highlights business portfolio, innovation, and actions to drive future performance 151
6.57 May 08, 2023: DURECT reports Q1 2023 financial results and business update 151
6.58 May 05, 2023: PAVmed to hold a business update conference call and webcast on May 17, 2023 152
6.59 May 04, 2023: Teleflex reports first quarter financial results and full year 2023 outlook 153
6.60 May 04, 2023: Cardinal Health reports third quarter fiscal year 2023 results and raises fiscal year 2023 non-gaap eps guidance 154
6.61 May 04, 2023: KORU Medical Systems announces 2023 first quarter financial results 156
6.62 Apr 28, 2023: Eitan Medical introduces new multi-therapy ambulatory infusion system 158
6.63 Apr 26, 2023: MicroPort announces annual results for 2022 158
6.64 Apr 25, 2023: Vial2Bag Advanced 20mm Admixture Device: Cost Reduction and operational efficiency in IV medication distribution 158
6.65 Apr 25, 2023: medaptus announces agreement with fresenius Kabi to offer hospitals financial reimbursement software for infusion services 159
6.66 Apr 25, 2023: 3M announces more layoffs 159
6.67 Apr 21, 2023: KORU Medical Systems to report first quarter financial results on May 4, 2023 159
6.68 Apr 20, 2023: Teleflex announces first quarter 2023 earnings conference call information 160
6.69 Apr 20, 2023: Alnylam to webcast conference call discussing first quarter 2023 financial results 160
6.70 Apr 17, 2023: Vizient Innovative Technology contract awarded to Fresenius Kabi for the Ivenix Infusion System 160
6.71 Apr 14, 2023: Baxter International receives additional 510(k) clearance for SIGMA Spectrum Infusion Pump with Master Drug Library 161
6.72 Apr 14, 2023: Baxter International receives additional 510(k) clearance for Spectrum IQ Infusion System With Dose IQ Safety Software 161
6.73 Apr 13, 2023: Lilly Confirms Date and Conference Call for First-Quarter 2023 Financial Results 161
6.74 Apr 12, 2023: Intera Oncology showcases Intera 3000 hepatic artery infusion (HAI) Pump at the Annual Cholangiocarcinoma Foundation Conference 162
6.75 Apr 12, 2023: Akoya Biosciences and enable medicine introduce cloud platform to power faster analysis of PhenoCycler-Fusion Data 162
6.76 Apr 12, 2023: Medtronic has a layoff in California 163
6.77 Apr 06, 2023: Alnylam Pharmaceuticals Publishes 2022 Annual Report 163
6.78 Apr 05, 2023: Dymax reveals MD 1045-M multipurpose adhesive for prefilled syringes and injection devices 163
6.79 Mar 31, 2023: Alimera Sciences Announces 2022 Financial Results and Business Update 163
6.80 Mar 29, 2023: Baxter to Host Annual Meeting of Stockholders in Virtual Format 166
6.81 Mar 22, 2023: AbbVie provides regulatory update on ABBV-951 (Foscarbidopa/Foslevodopa) New Drug Application 166
6.82 Mar 22, 2023: ConvaTec Group Notice of Annual General Meeting 166
6.83 Mar 16, 2023: Intera Oncology relaunches Hepatic Artery Infusion (HAI) pump, restoring powerful treatment option for patients fighting metastatic cancer in the liver 167
6.84 Mar 16, 2023: Medtronic joins in $25M funding round for home monitoring company 168
6.85 Mar 14, 2023: PAVmed Provides Business Update and Preliminary Fourth Quarter and Full Year 2022 Financial Results 168
6.86 Mar 09, 2023: ConvaTec Group Announces Annual Results for the twelve months ended 31 December 2022 170
6.87 Mar 08, 2023: Alnylam Announces Appointment of Peter Kellogg to Board of Directors 172
6.88 Mar 08, 2023: KORU Medical Systems Announces 2022 Q4 and Full Year Financial Results, Accelerating to 19% Revenue Growth in 2022 172
6.89 Feb 24, 2023: IRADIMED issues Urgent Medical Device Correction 174
6.90 Feb 23, 2023: Teleflex Reports Fourth Quarter and Full Year 2022 Financial Results 174
6.91 Feb 23, 2023: Alnylam Pharmaceuticals Reports Fourth Quarter and Full Year 2022 Financial Results and Highlights Recent Period Activity 178
6.92 Feb 22, 2023: EryDel provides regulatory update on EryDex for the treatment of Ataxia Telangiectasia 181
6.93 Feb 22, 2023: Novo Nordisk Notice for the Annual General Meeting 182
6.94 Feb 22, 2023: KORU Medical Systems to Report Fourth Quarter and Full Year 2022 Financial Results on March 8, 2023 182
6.95 Feb 21, 2023: Teleflex to Present at the 44th Annual Raymond James Institutional Investors Conference 183
6.96 Feb 09, 2023: Teleflex Announces Fourth Quarter 2022 Earnings Conference Call Information 183
6.97 Feb 09, 2023: Baxter Reports Fourth-Quarter and Full-Year 2022 Results 183
6.98 Feb 09, 2023: Terumo Notice Concerning Revision of the Full-Year Financial Guidance for Fiscal Year Ending March 31, 2023 186
6.99 Feb 09, 2023: Terumo Corp Announces Consolidated Financial Results for the Third Quarter Ended December 31, 2022 186
6.100 Feb 03, 2023: Moog Reports First Quarter 2023 Results With Sales Growth and Improving Margins 187
6.101 Feb 02, 2023: Cardinal Health Reports Second Quarter Fiscal Year 2023 Results and Raises Fiscal Year 2023 Non-GAAP EPS Guidance 191
6.102 Jan 31, 2023: Daiken Medical Announces Summary of Non-Consolidated Financial Results for the Nine Months Ended December 31, 2022 192
6.103 Jan 25, 2023: KORU Medical Systems Announces Subcutaneous Immunoglobulin Prefilled Syringe Development Agreement 192
6.104 Jan 24, 2023: 3M Reports Fourth-Quarter and Full-Year 2022 Results 193
6.105 Jan 24, 2023: 3M Lays off 2500 employees 195
6.106 Jan 19, 2023: Intera 3000, the only FDA-Approved Implantable Pump for HAI Therapy, showcased at ASCO Gastrointestinal Cancer Symposium 2023 195
6.107 Jan 19, 2023: Lilly Confirms Date and Conference Call for Fourth-Quarter 2022 Financial Results 195
6.108 Jan 10, 2023: Fresenius Kabi’s Ivenix infusion system goes live at San Luis Valley Health 196
6.109 Dec 23, 2022: Medical Device Company Zyno Medical LLC Agrees To Pay Nearly $500,000 To Resolve False Claims Act Allegations Relating to Defective Medical Device 196
6.110 Dec 19, 2022: Cardinal Health Names Aaron Alt Chief Financial Officer 198
6.111 Dec 08, 2022: Edwards Lifesciences Announces CEO Succession Plan 198
6.112 Dec 08, 2022: Edwards Lifesciences Announces CEO Succession Plan 199
7 Appendix 201
7.1 Methodology 201
7.2 About GlobalData 204
7.3 Contact Us 204
7.4 Disclaimer 204
![]()