Interventional Radiology Devices Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2021
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Interventional Radiology which generates high quality image of tumour by performing simultaneous motion estimation and motion-compensated image reconstruction for cancer patients undergoing radiation therapy. The report provides comprehensive information about the Interventional Radiology Devices pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
What are the market dynamics in the interventional radiology replacement devices pipeline products market?
As of November 2021, 25 pipeline products were in the clinical stage and 17 in the inactive stage. 5 of the pipeline products were in the approval process and 2 in the pre-clinical stage. 14 pipeline products were in the early development stage. The US was found to be the leader in interventional radiology replacement devices pipeline products market followed by Canada, Europe, China, Costa Rica, El Salvador, Mexico, Russia, South Korea, Israel, Ecuador, Chile, Argentina, and Brazil. There were 20 ongoing, recruiting trials of interventional radiology replacement devices pipeline products and 4 were ongoing, not recruiting.
Which are the key players in the interventional radiology replacement devices pipeline products market?
The key players in the market are Accuray Inc, Actuality Medical, Inc. (Inactive), Akesis LLC, Bendit Technologies Ltd, Biocompatibles UK Ltd, Boston Scientific Corp, BrachyFoam LLC, Cornell University, CR Bard Inc and Creo Medical Ltd.
Company overview:
Accuray Inc: Accuray Inc (Accuray) is a medical device company which focuses on the development, manufacture, and sale of personalized treatment solutions for radiosurgery, stereotactic body radiation therapy, intensity modulated radiation therapy, image guided radiation therapy and adaptive radiation therapy. The company’s major products include CyberKnife robotic radiosurgery systems, TomoTherapy system, Radixact delivery treatment platform and Software Solutions. Its flagship product, CyberKnife robotic radiosurgery system is designed to treat tumors anywhere in the body non-invasively with an accuracy of sub-millimeter. TomoTherapy system combines a CT scanner with a radiation treatment delivery system that is used by healthcare professionals in the treatment of a wide range of cancer types. Its major services include post-contract customer support, training, installation services, and other professional services. The company sells its products in the US and through distributors in Latin America, Europe, and Asia. Accuray is headquartered in Sunnyvale, California, the US.
Its product is Linac (Linear Accelerator) that is intended to be used during radiation therapy procedures. It is designed to deliver high-energy x-rays or electrons to the region of the patient’s tumor.
Akesis LLC: Akesis LLC (Akesis) is a contract research organization that provides clinical research services and regulatory affairs consulting. The company offers pharmaceutical development services such as feasibility and protocol development, project and program management, clinical trial monitoring, oversight and conduct of phase i-iv trials, site identification and selection, patient recruitment support, and regulatory document management. It caters its services to therapeutic areas such as dermatology and topical, epilepsy, pediatrics, ophthalmology, osteoarthritis, ulcerative colitis, and women’s and men’s health.
Its product is Akesis Gemini 360 that is intended for stereotactic radiotherapy. It is designed to deliver radiotherapy with integrated real-time imaging with 360° rotation. It is based on Continuous 360° Rotational Technology. It consists of a MV LINAC head and 120 leaf MLC.
Boston Scientific Corp: Boston Scientific Corp (Boston Scientific) is a medical technology company that develops, manufactures, and commercializes devices for a range of interventional medical specialties. The company offers products in the areas of electrophysiology, gastroenterology, gastrointestinal surgery, female pelvic medicine, gynecology, interventional cardiology, interventional radiology, neurological surgery, orthopedic surgery, pain medicine, pulmonology, urology, and vascular surgery. Boston Scientific serves hospitals, clinics, outpatient facilities and medical offices across the world. The company has manufacturing facilities in the US, Ireland, Costa Rica, Brazil, Malaysia, Puerto Rico, and Switzerland. It sells products directly and also through a network of distributors and dealers in Europe, the Middle East, Africa, Asia-Pacific, and the Americas. Boston Scientific is headquartered in Marlborough, Massachusetts, the US.
Its product is, TheraSphere – Brain Cancer is a radioembolization device intended for the treatment of brain cancer. It is designed to deliver a radiotherapeutic agent (yttrium-90) directly to the tumor via blood flow. It consists of millions of microscopic, insoluble glass microspheres being infused into the arteries that feed tumors. Diameter (mm): 20-30 Delivers Yttrium-90.
Cornell University: Cornell University (CU), subsidiary of State University of New York, is provider of education services. The university offers education and research services. It offers educational services of undergraduates, graduate, and professional courses. CU provides education in the areas environmental engineering and biological engineering, crop and soil sciences, communication, and development sociology departments. The university operates schools and colleges. It offers research services with multidisciplinary partnerships and for graduates and undergraduates. The university has labs, facilities, centers, and institutes for research. It has services in the US, Italy, and Qatar. CU is headquartered at Ithaca, New York, the US.
Its product is, safety catheter that is intended to be used during interventional radiology and endovascular procedures. It is designed to facilitate delivery of guidewires without dilators and/or sheath. It comprises a soft, atraumatic lumen portion and has a temperature-sensitive exoskeleton.
CR Bard Inc: CR Bard Inc (CR Bard), a subsidiary of Becton Dickinson and Co, is a medical technology company that develops and markets medical devices in the areas of urology, vascular, oncology and surgical specialty. Its offerings include foley catheters, foley trays, irrigation trays, collection systems, intermittent catheters, and trays. CR Bard is headquartered in Murray Hill, New Jersey, the US. Its product is, Loc Wire Replacement that is a signal transmission device designed for breast cancer detection. It is designed to detect the abnormal spot in the breast.
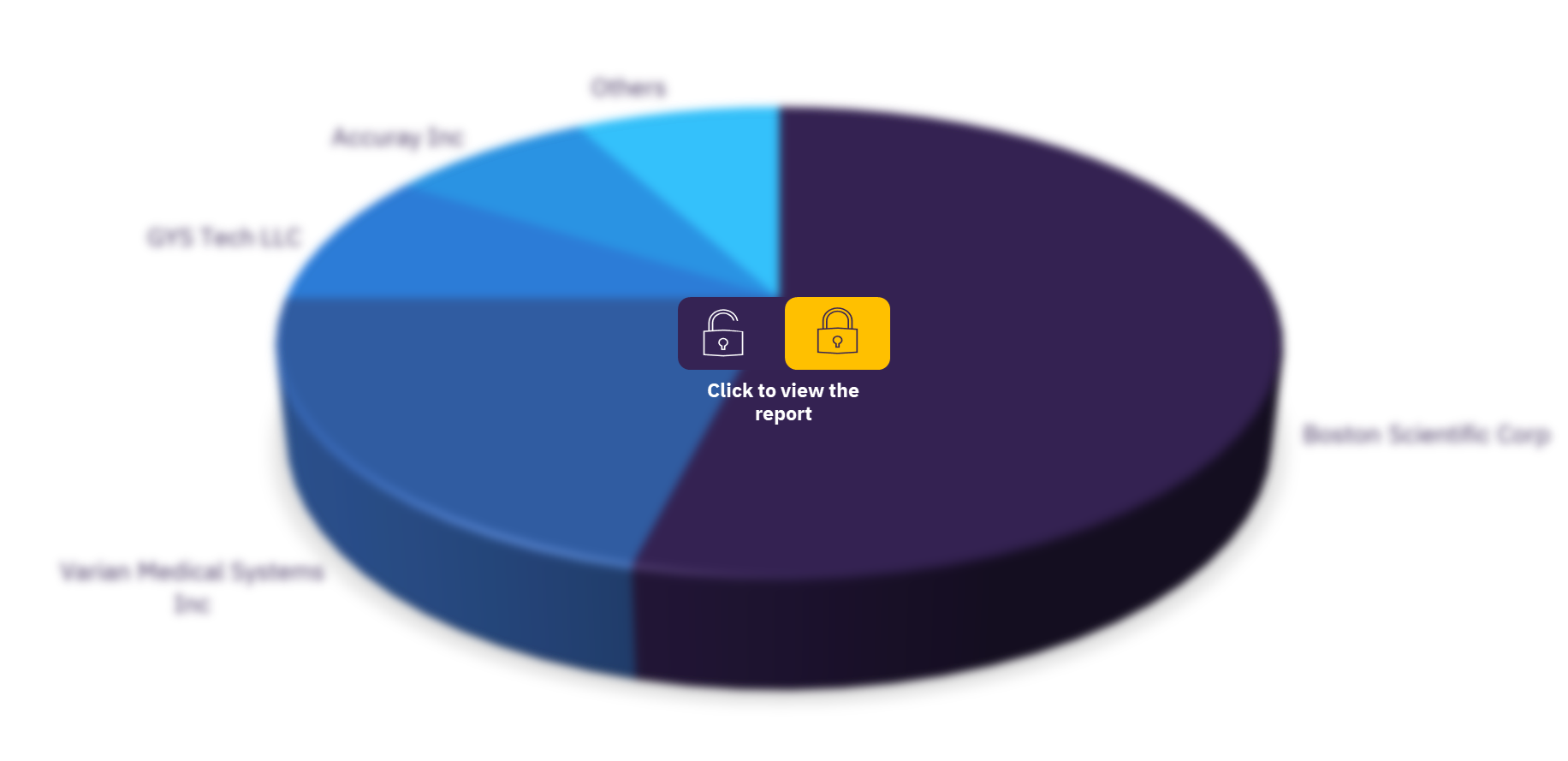
Interventional radiology replacement devices pipeline products, by key players
To know more about key players, download a free report sample
Market report scope
| Key countries/regions | The US, Canada, Europe, China, Costa Rica, El Salvador, Mexico, Russia, South Korea, Israel, Ecuador, Chile, Argentina, and Brazil |
| Key players | Accuray Inc, Actuality Medical, Inc. (Inactive), Akesis LLC, Bendit Technologies Ltd, Biocompatibles UK Ltd, Boston Scientific Corp, BrachyFoam LLC, Cornell University, CR Bard Inc, Creo Medical Ltd, Elekta AB, GYS Tech LLC, IMRIS, Inc., Intelligent Ultrasound plc, Landauer Inc, MagnetTx Oncology Solutions Inc, Medical Device Investments, Inc., Neuboron Medtech Ltd, Protonvda Inc, Quirem Medical BV, Radiabeam Technologies LLC, Radiaction Ltd, RadiaDyne, LLC, Raysearch Laboratories AB, Siemens Healthineers AG, SpectraCure AB, Standard Imaging Inc, TheraBionic LLC, Theralase Technologies Inc, University Health Network, University of California Los Angeles, University of Florida, University of South Florida, University of Texas Southwestern Medical Center, Varian Medical Systems Inc, Vivos Inc, XL Sci-Tech Inc, Xoft Inc, and ZHealth Documentation LLC. |
Scope
- Extensive coverage of the Interventional Radiology Devices under development.
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities.
- The report reviews the major players involved in the development of Interventional Radiology Devices and list all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from Early Development to the Approved/Issued stage.
- The report provides key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage.
- Identify and understand important and diverse types of Interventional Radiology Devices under development.
- Develop market-entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Actuality Medical, Inc. (Inactive)
Akesis LLC
Bendit Technologies Ltd
Biocompatibles UK Ltd
Boston Scientific Corp
BrachyFoam LLC
Cornell University
CR Bard Inc
Creo Medical Ltd
Elekta AB
GYS Tech LLC
IMRIS, Inc.
Intelligent Ultrasound plc
Landauer Inc
MagnetTx Oncology Solutions Inc
Medical Device Investments, Inc.
Neuboron Medtech Ltd
Protonvda Inc
Quirem Medical BV
Radiabeam Technologies LLC
Radiaction Ltd
RadiaDyne, LLC
Raysearch Laboratories AB
Siemens Healthineers AG
SpectraCure AB
Standard Imaging Inc
TheraBionic LLC
Theralase Technologies Inc
University Health Network
University of California Los Angeles
University of Florida
University of South Florida
University of Texas Southwestern Medical Center
Varian Medical Systems Inc
Vivos Inc
XL Sci-Tech Inc
Xoft Inc
ZHealth Documentation LLC
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key geographic regions in the interventional radiology replacement devices pipeline products market?
The US was leading the interventional radiology replacement devices pipeline products market followed by Canada, Europe, China, Costa Rica, El Salvador, Mexico, Russia, South Korea, Israel, Ecuador, Chile, Argentina, and Brazil.
-
Which are the key players in the interventional radiology replacement devices pipeline products market?
The key players in the market are Accuray Inc, Actuality Medical, Inc. (Inactive), Akesis LLC, Bendit Technologies Ltd, Biocompatibles UK Ltd, Boston Scientific Corp, BrachyFoam LLC, Cornell University, CR Bard Inc and Creo Medical Ltd.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Medical Devices reports