Leiomyosarcoma Drugs in Development by Stages, Target, MoA, RoA, Molecule Type and Key Players, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Leiomyosarcoma is an aggressive soft tissue sarcoma derived from smooth muscle cells typically of uterine, gastrointestinal or soft tissue origin. Symptoms include a noticeable lump or swelling, pain, if it presses on nerves or muscles and a blockage in the stomach or intestines or gastrointestinal bleeding if the tumor is located in the abdomen or digestive tract. Predisposing factors include age, chemical exposure and radiation exposure.
The Leiomyosarcoma – drugs in development research report provides a comprehensive overview on the therapeutics under development for Leiomyosarcoma, complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. The report also covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and press releases. Additionally, the report provides an overview of key players involved in therapeutic development for Leiomyosarcoma and features dormant and discontinued projects.
| Key Targets | Vascular Endothelial Growth Factor Receptor 2, Programmed Cell Death 1 Ligand 1, Programmed Cell Death Protein 1, Vascular Endothelial Growth Factor Receptor 3, Mast/Stem Cell Growth Factor Receptor Kit, Cells Expressing Ganglioside GD2, Cells Expressing Latent Membrane Protein 1, Cells Expressing Latent Membrane Protein 2, Exportin 1, Fibroblast Growth Factor Receptor, Leukocyte Surface Antigen CD47, Platelet Derived Growth Factor Receptor Beta |
| Key Mechanisms of action | Vascular Endothelial Growth Factor Receptor 2 Inhibitor, Programmed Cell Death 1 Ligand 1 Inhibitor, Programmed Cell Death Protein 1 Antagonist, Vascular Endothelial Growth Factor Receptor 3 Inhibitor, Mast/Stem Cell Growth Factor Receptor Kit Inhibitor, Cytotoxic To Cells Expressing Ganglioside GD2, Cytotoxic To Cells Expressing Latent Membrane Protein 1 , Cytotoxic To Cells Expressing Latent Membrane, Protein 2, Exportin 1 Inhibitor, and Fibroblast Growth Factor Receptor Inhibitor |
| Key Routes of Administration | Intravenous, Oral, Subcutaneous, Intratumor, Intravenous Drip, Intravesical, Parenteral, Topical, Intradermal, and Intralesional |
| Key molecule types | Small Molecule, Monoclonal Antibody, Cell Therapy, Fusion Protein, Biologic, Gene-Modified Cell Therapy, Inactivated Vaccine, Monoclonal Antibody Conjugated, and Recombinant Peptide |
| Major companies | Advenchen Laboratories LLC, AstraZeneca Plc, Pharma Mar SA, Karyopharm Therapeutics Inc, Merck KGaA, Pfizer Inc, 3SBio Inc, Actuate Therapeutics Inc, Agenus Inc, Alphamab Oncology, ALX Oncology Holdings Inc, Apexigen Inc, and APIM Therapeutics AS |
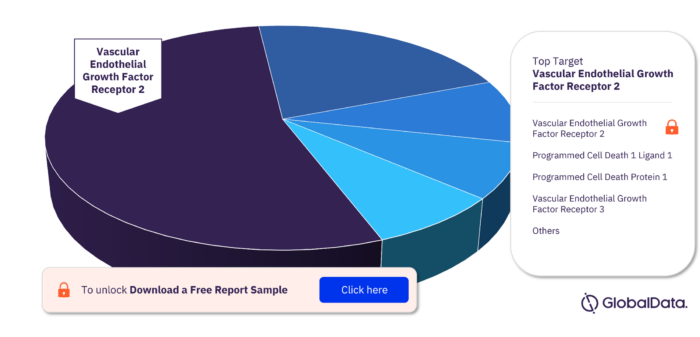
Key Targets in the Leiomyosarcoma Pipeline Drugs Market
In the Leiomyosarcoma pipeline drugs market the key targets are Vascular Endothelial Growth Factor Receptor 2, Programmed Cell Death 1 Ligand 1, Programmed Cell Death Protein 1, Vascular Endothelial Growth Factor Receptor 3, Mast/Stem Cell Growth Factor Receptor Kit, Cells Expressing Ganglioside GD2, Cells Expressing Latent Membrane Protein 1, Cells Expressing Latent Membrane Protein 2, Exportin 1, Fibroblast Growth Factor Receptor, Leukocyte Surface Antigen CD47, Platelet Derived Growth Factor Receptor Beta. Vascular Endothelial Growth Factor Receptor 2 has the highest number of pipeline products.
Leiomyosarcoma Pipeline Drugs Market, by Targets
For more target insights, download a free report sample
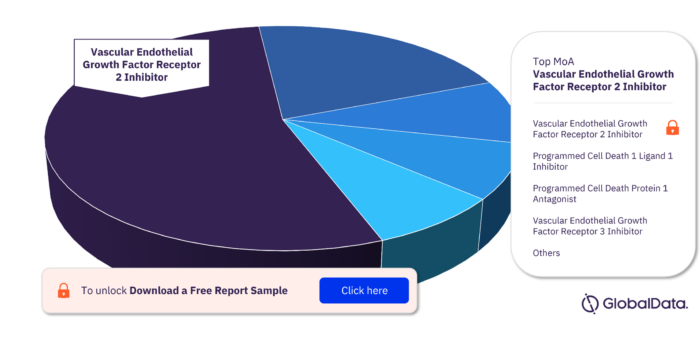
Key MoA in the Leiomyosarcoma Pipeline Drugs Market
Vascular Endothelial Growth Factor Receptor 2 Inhibitor has the highest number of pipeline products followed by Programmed Cell Death 1 Ligand 1 Inhibitor, Programmed Cell Death Protein 1 Antagonist, Vascular Endothelial Growth Factor Receptor 3 Inhibitor, Mast/Stem Cell Growth Factor Receptor Kit Inhibitor, Cytotoxic To Cells Expressing Ganglioside GD2, Cytotoxic To Cells Expressing Latent Membrane Protein 1 , Cytotoxic To Cells Expressing Latent Membrane, Protein 2, Exportin 1 Inhibitor, and Fibroblast Growth Factor Receptor Inhibitor.
Leiomyosarcoma Pipeline Drugs Market, by MoA
To get more insights on key MoA, download a free sample report
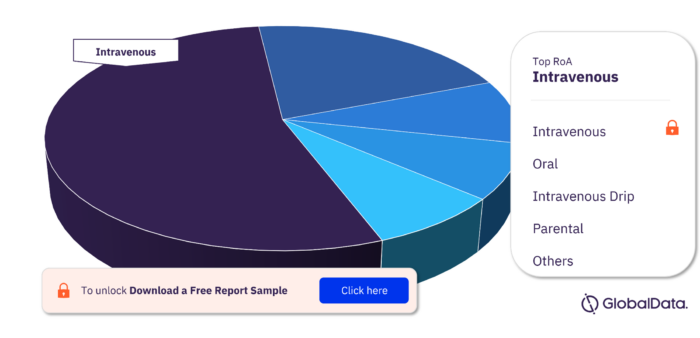
Leiomyosarcoma Pipeline Drugs Market Segmentation by RoA
The key routes of administration in the Leiomyosarcoma pipeline drugs market are Intravenous, Oral, Subcutaneous, Intratumor, Intravenous Drip, Intravesical, Parenteral, Topical, Intradermal, and Intralesional. Intravenous has the maximum number of pipeline products.
Leiomyosarcoma Pipeline Drugs Market Analysis, by RoA
To get more insights on key RoA, download a free sample report
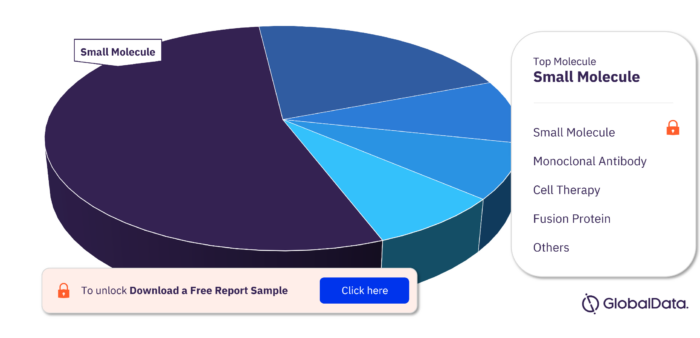
Key Molecule Types in the Leiomyosarcoma Pipeline Drugs Market
In the Leiomyosarcoma pipeline drugs market the key molecule types are Small Molecule, Monoclonal Antibody, Cell Therapy, Fusion Protein, Biologic, Gene-Modified Cell Therapy, Inactivated Vaccine, Monoclonal Antibody Conjugated, and Recombinant Peptide.
Leiomyosarcoma Pipeline Drugs Market, by Molecule Types
To get more insights on key molecule types, download a free sample report
Major Companies in the Leiomyosarcoma Pipeline Drugs Market
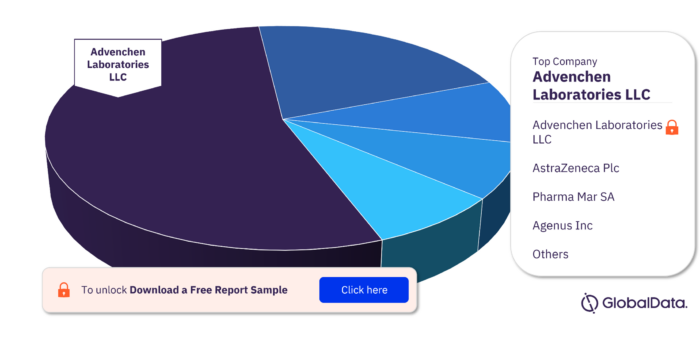
The major companies in the Leiomyosarcoma pipeline drugs market are Advenchen Laboratories LLC, AstraZeneca Plc, Pharma Mar SA, Karyopharm Therapeutics Inc, Merck KGaA, Pfizer Inc, 3SBio Inc, Actuate Therapeutics Inc, Agenus Inc, Alphamab Oncology, ALX Oncology Holdings Inc, Apexigen Inc, and APIM Therapeutics AS.
Advenchen Laboratories LLC: It is a pharmaceutical company that offers healthcare solutions. The company conducts pharmaceutical research and develops small molecule cancer drug discovery programs. It provides pipeline such as angiogenesis inhibitors and small molecule protein tyrosine kinases inhibitors, among others. Advenchen Laboratories provides services such as producing inhibitors for the treatment of human colon cancer, liver cancer, gastric cancer, lungs and breast cancer, among others. Advenchen Laboratories also provides customized chemical intermediate services. The company operates in China and the US. Advenchen Laboratories is headquartered in Moorpark, California, the US.
Leiomyosarcoma Pipeline Drugs Market, by Major Companies
For more company insights, download a free sample report
Leiomyosarcoma Pipeline Drugs Market Overview
| Key Targets | Vascular Endothelial Growth Factor Receptor 2, Programmed Cell Death 1 Ligand 1, Programmed Cell Death Protein 1, Vascular Endothelial Growth Factor Receptor 3, Mast/Stem Cell Growth Factor Receptor Kit, Cells Expressing Ganglioside GD2, Cells Expressing Latent Membrane Protein 1, Cells Expressing Latent Membrane Protein 2, Exportin 1, Fibroblast Growth Factor Receptor, Leukocyte Surface Antigen CD47, Platelet Derived Growth Factor Receptor Beta |
| Key Mechanisms of action | Vascular Endothelial Growth Factor Receptor 2 Inhibitor, Programmed Cell Death 1 Ligand 1 Inhibitor, Programmed Cell Death Protein 1 Antagonist, Vascular Endothelial Growth Factor Receptor 3 Inhibitor, Mast/Stem Cell Growth Factor Receptor Kit Inhibitor, Cytotoxic To Cells Expressing Ganglioside GD2, Cytotoxic To Cells Expressing Latent Membrane Protein 1 , Cytotoxic To Cells Expressing Latent Membrane, Protein 2, Exportin 1 Inhibitor, and Fibroblast Growth Factor Receptor Inhibitor |
| Key Routes of Administration | Intravenous, Oral, Subcutaneous, Intratumor, Intravenous Drip, Intravesical, Parenteral, Topical, Intradermal, and Intralesional |
| Key molecule types | Small Molecule, Monoclonal Antibody, Cell Therapy, Fusion Protein, Biologic, Gene-Modified Cell Therapy, Inactivated Vaccine, Monoclonal Antibody Conjugated, and Recombinant Peptide |
| Major companies | Advenchen Laboratories LLC, AstraZeneca Plc, Pharma Mar SA, Karyopharm Therapeutics Inc, Merck KGaA, Pfizer Inc, 3SBio Inc, Actuate Therapeutics Inc, Agenus Inc, Alphamab Oncology, ALX Oncology Holdings Inc, Apexigen Inc, and APIM Therapeutics AS |
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of Leiomyosarcoma
- The pipeline guide reviews pipeline therapeutics for Leiomyosarcoma by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in Leiomyosarcoma therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates Leiomyosarcoma therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA), and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews the latest news related to pipeline therapeutics for Leiomyosarcoma
Scope
- The pipeline guide provides a snapshot of the global therapeutic landscape of Leiomyosarcoma
- The pipeline guide reviews pipeline therapeutics for Leiomyosarcoma by companies and universities/research institutes based on information derived from company and industry-specific sources.
- The pipeline guide covers pipeline products based on several stages of development ranging from pre-registration till discovery and undisclosed stages.
- The pipeline guide features descriptive drug profiles for the pipeline products which comprise, product description, descriptive licensing and collaboration details, R&D brief, MoA & other developmental activities.
- The pipeline guide reviews key companies involved in Leiomyosarcoma therapeutics and enlists all their major and minor projects.
- The pipeline guide evaluates Leiomyosarcoma therapeutics based on mechanism of action (MoA), drug target, route of administration (RoA), and molecule type.
- The pipeline guide encapsulates all the dormant and discontinued pipeline projects.
- The pipeline guide reviews the latest news related to pipeline therapeutics for Leiomyosarcoma
Reasons to Buy
- Procure strategically important competitor information, analysis, and insights to formulate effective R&D strategies.
- Recognize emerging players with a potentially strong product portfolio and create effective counter strategies to gain a competitive advantage.
- Find and recognize significant and varied types of therapeutics under development for Leiomyosarcoma
- Classify potential new clients or partners in the target demographic.
- Develop tactical initiatives by understanding the focus areas of leading companies.
- Plan mergers and acquisitions meritoriously by identifying key players and their most promising pipeline therapeutics.
- Formulate corrective measures for pipeline projects by understanding Leiomyosarcoma pipeline depth and focus of Indication therapeutics.
- Develop and design in-licensing and out-licensing strategies by identifying prospective partners with the most attractive projects to enhance and expand business potential and scope.
- Adjust the therapeutic portfolio by recognizing discontinued projects and understand from the know-how what drove them from the pipeline.
Actuate Therapeutics Inc
Advenchen Laboratories LLC
Agenus Inc
Alphamab Oncology
ALX Oncology Holdings Inc
Apexigen Inc
APIM Therapeutics AS
Apollomics Inc
AstraZeneca Plc
Atara Biotherapeutics Inc
BeiGene Ltd
BioAtla Inc
BioMed Valley Discoveries Inc
Calithera Biosciences Inc
Cebiotex SL
Clovis Oncology Inc
CStone Pharmaceuticals Co Ltd
Eli Lilly and Co
ENB Therapeutics LLC
EUSA Pharma (UK) Ltd
Exelixis Inc
F. Hoffmann-La Roche Ltd
Immix BioPharma Inc
Immodulon Therapeutics Ltd
Incyte Corp
Karyopharm Therapeutics Inc
Kuur Therapeutics Ltd
Merck & Co Inc
Merck KGaA
Ono Pharmaceutical Co Ltd
Pfizer Inc
Pharma Mar SA
Philogen SpA
PTC Therapeutics Inc
Shanghai De Novo Pharmatech Co Ltd
Shanghai Junshi Bioscience Co Ltd
Tactical Therapeutics Inc
Tempest Therapeutics Inc
Tessa Therapeutics Ltd
Viracta Therapeutics Inc
Y-mAbs Therapeutics Inc
Yooyoung Pharm Co Ltd
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key targets in the Leiomyosarcoma pipeline drugs market?
In the Leiomyosarcoma pipeline drugs market the key targets are Vascular Endothelial Growth Factor Receptor 2, Programmed Cell Death 1 Ligand 1, Programmed Cell Death Protein 1, Vascular Endothelial Growth Factor Receptor 3, Mast/Stem Cell Growth Factor Receptor Kit, Cells Expressing Ganglioside GD2, Cells Expressing Latent Membrane Protein 1, Cells Expressing Latent Membrane Protein 2, Exportin 1, Fibroblast Growth Factor Receptor, Leukocyte Surface Antigen CD47, Platelet Derived Growth Factor Receptor Beta.
-
What are the key mechanisms of action in the Leiomyosarcoma pipeline drugs market?
In the Leiomyosarcoma pipeline drugs market the key mechanisms of action are Vascular Endothelial Growth Factor Receptor 2 Inhibitor, Programmed Cell Death 1 Ligand 1 Inhibitor, Programmed Cell Death Protein 1 Antagonist, Vascular Endothelial Growth Factor Receptor 3 Inhibitor, Mast/Stem Cell Growth Factor Receptor Kit Inhibitor, Cytotoxic To Cells Expressing Ganglioside GD2, Cytotoxic To Cells Expressing Latent Membrane Protein 1 , Cytotoxic To Cells Expressing Latent Membrane, Protein 2, Exportin 1 Inhibitor, and Fibroblast Growth Factor Receptor Inhibitor.
-
What are the key routes of administration in the Leiomyosarcoma pipeline drugs market?
The key routes of administration in the Leiomyosarcoma pipeline drugs market are Intravenous, Oral, Subcutaneous, Intratumor, Intravenous Drip, Intravesical, Parenteral, Topical, Intradermal, and Intralesional.
-
What are the key molecule types in the Leiomyosarcoma pipeline drugs market?
The key molecule types in the Leiomyosarcoma pipeline drugs market are Small Molecule, Monoclonal Antibody, Cell Therapy, Fusion Protein, Biologic, Gene-Modified Cell Therapy, Inactivated Vaccine, Monoclonal Antibody Conjugated, and Recombinant Peptide.
-
What are the major companies in the Leiomyosarcoma pipeline drugs market?
In the Leiomyosarcoma pipeline drugs market the major companies are Advenchen Laboratories LLC, AstraZeneca Plc, Pharma Mar SA, Karyopharm Therapeutics Inc, Merck KGaA, Pfizer Inc, 3SBio Inc, Actuate Therapeutics Inc, Agenus Inc, Alphamab Oncology, ALX Oncology Holdings Inc, Apexigen Inc, and APIM Therapeutics AS.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Oncology reports