Neurological Diagnostic and Monitoring Equipment – Pipeline Products by Stage of Development 41
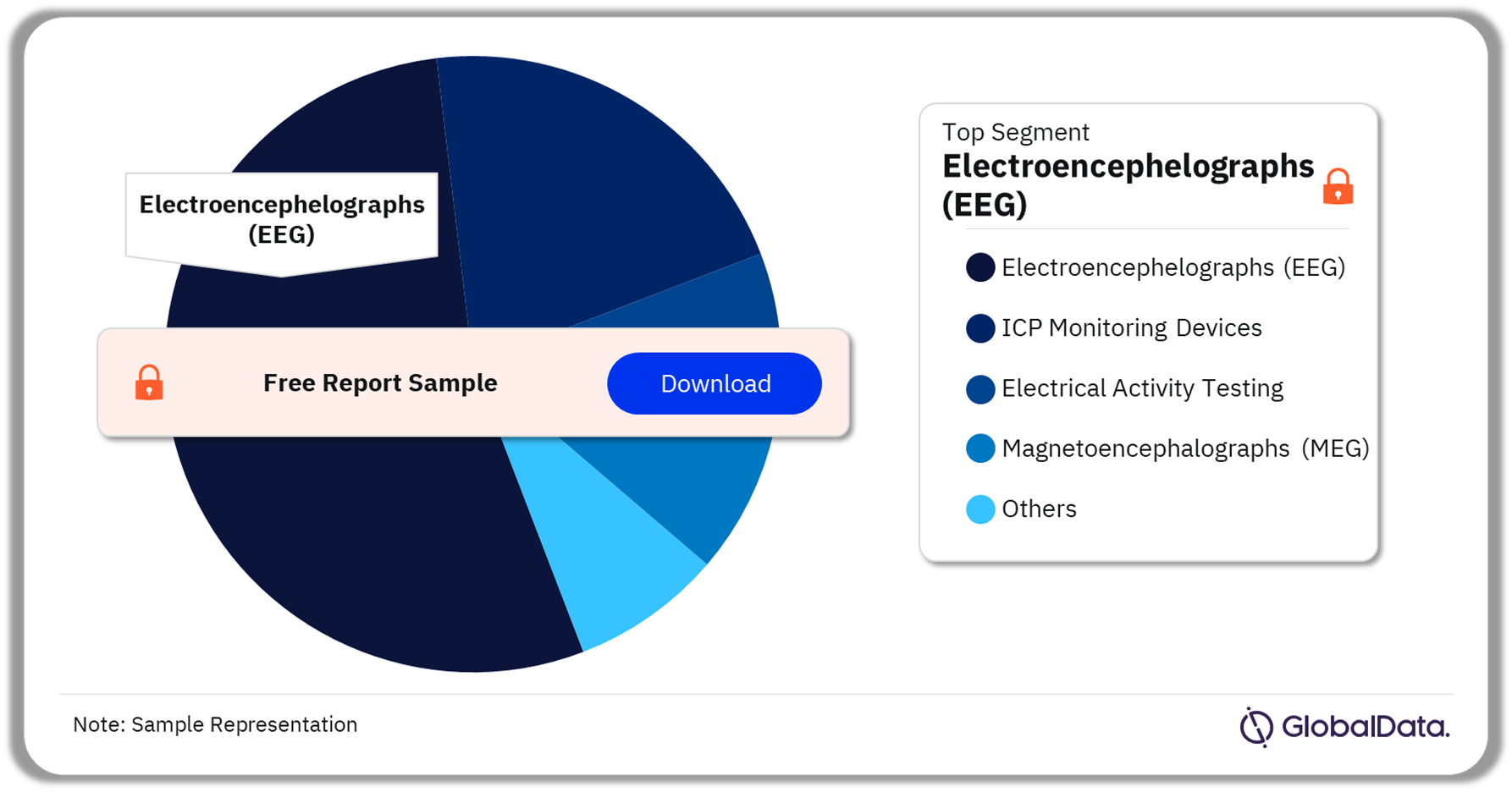
Neurological Diagnostic and Monitoring Equipment – Pipeline Products by Segment 42
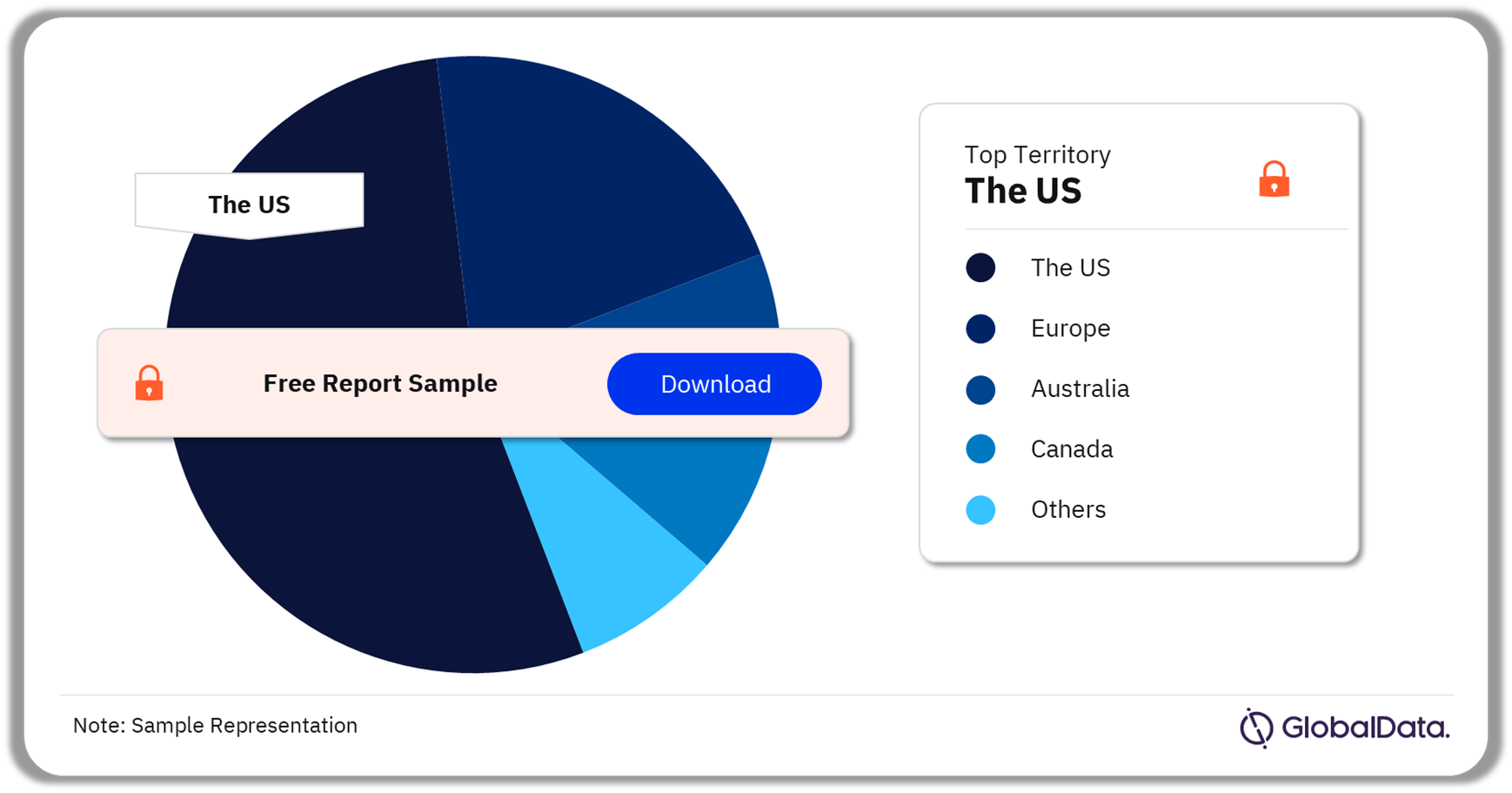
Neurological Diagnostic and Monitoring Equipment – Pipeline Products by Territory 43
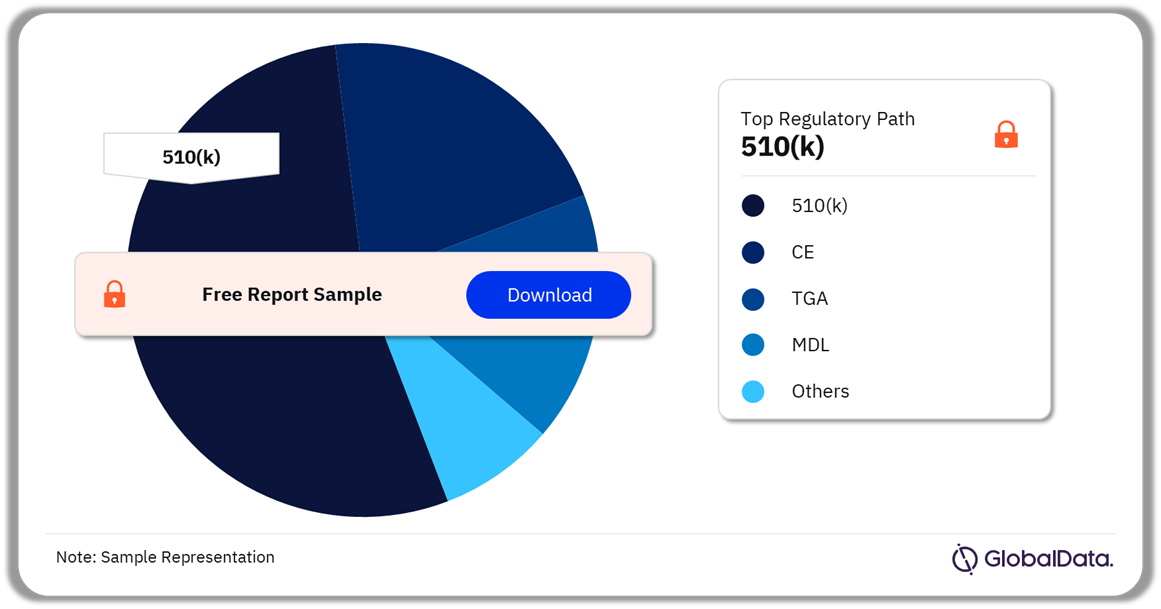
Neurological Diagnostic and Monitoring Equipment – Pipeline Products by Regulatory Path 44
Neurological Diagnostic and Monitoring Equipment – Pipeline Products by Estimated Approval Date 45
Neurological Diagnostic and Monitoring Equipment – Ongoing Clinical Trials 46
Neurological Diagnostic and Monitoring Equipment Companies – Pipeline Products by Stage of Development 47
Neurological Diagnostic and Monitoring Equipment – Pipeline Products by Stage of Development 58
Aalto University Pipeline Products & Ongoing Clinical Trials Overview 68
MEG-MRI Device – Product Status 68
MEG-MRI Device – Product Description 68
AAT Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 69
Mente Autism – Product Status 69
Mente Autism – Product Description 69
AAT Medical Ltd – Ongoing Clinical Trials Overview 70
Mente Autism – Phase I/II Study to Assess the Safety and the Efficacy of the Novel NTI/Dolce Strains in a Paediatric Population, along with the Efficacy of the Mente Device When Used in Combination with the Novel NTI/Dolce Medicinal Cannabis Strains 71
ADM Diagnostics LLC Pipeline Products & Ongoing Clinical Trials Overview 72
ADMdx – Alzheimer’s Disease – Product Status 72
ADMdx – Alzheimer’s Disease – Product Description 72
Ad-Tech Medical Instrument Corp Pipeline Products & Ongoing Clinical Trials Overview 73
Diagnostic Device – Brain Disorders – Product Status 73
Diagnostic Device – Brain Disorders – Product Description 73
Diagnostic Device – Trauma – Product Status 74
Diagnostic Device – Trauma – Product Description 74
ICP Monitoring System – Product Status 74
ICP Monitoring System – Product Description 75
Advanced Diamond Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 76
Chronically Implantable UNCD Microarray Electrode – Product Status 76
Chronically Implantable UNCD Microarray Electrode – Product Description 76
Advanced Medical Electronics Corp Pipeline Products & Ongoing Clinical Trials Overview 77
POC aEEG Monitor – Product Status 77
POC aEEG Monitor – Product Description 77
Advanced Neurometrics Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 78
ANI-SI EEG System – Product Status 78
ANI-SI EEG System – Product Description 78
ANSwers Neuroscience Inc Pipeline Products & Ongoing Clinical Trials Overview 79
Diagnostic Device – Concussion – Product Status 79
Diagnostic Device – Concussion – Product Description 79
Arogya Medtech Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 80
CEREBROS – Product Status 80
CEREBROS – Product Description 80
MindEye – Product Status 81
MindEye – Product Description 81
Autonomix Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 82
Catheter-Based Microchip System – Product Status 82
Catheter-Based Microchip System – Product Description 82
Averia Health Solutions Pipeline Products & Ongoing Clinical Trials Overview 83
Concussion Screening And Management System – Product Status 83
Concussion Screening And Management System – Product Description 83
Avertus Inc Pipeline Products & Ongoing Clinical Trials Overview 84
10 Channel HALO Configuration Dry Wireless Ambulatory Device – Product Status 84
10 Channel HALO Configuration Dry Wireless Ambulatory Device – Product Description 84
Axem Neurotechnology Inc Pipeline Products & Ongoing Clinical Trials Overview 85
Axem Home – Product Status 85
Axem Home – Product Description 85
Axem Neurotechnology Inc – Ongoing Clinical Trials Overview 86
Axem Home – Portable Method of Motor Rehabilitation Using Functional Near-InfraRed Spectroscopy- Based Brain-computer-interface to Augment Post-stroke Recovery (fNIRS-PROMOTE- Recovery) 87
Axion BioSystems Inc Pipeline Products & Ongoing Clinical Trials Overview 88
Microneedle Array System – Product Status 88
Microneedle Array System – Product Description 88
Barron Associates, Inc. Pipeline Products & Ongoing Clinical Trials Overview 89
DASSO – Product Status 89
DASSO – Product Description 89
GEMINI System – Product Status 90
GEMINI System – Product Description 90
Bionics Institute Pipeline Products & Ongoing Clinical Trials Overview 91
Brain Activity Monitoring Device – Product Status 91
Brain Activity Monitoring Device – Product Description 91
Bioscan Research Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 92
Next Generation Cerebo – Product Status 92
Next Generation Cerebo – Product Description 92
Bioscan Research Pvt Ltd – Ongoing Clinical Trials Overview 93
Next Generation Cerebo – Assessment of Accuracy, Precision, and Feasibility of a near Infrared Light Device to Detect and Categorize the Intracranial Hematoma in Patients with Suspected Brain Injury Compared to Head CT Scan as the Gold Standard: A Prospective Study 94
Next Generation Cerebo – Assessment of Accuracy, Precision, and Feasibility of CEREBO – a near Infrared Light Device to Detect and Categorize the Intracranial Hematoma in Patients with Suspected Brain Injury Compared to Head Ct Scan as the Gold Standard: A Prospective Study 94
Next Generation Cerebo – Summative Usability Study of a Novel Device – CEREBO for Non Invasive Detection of Intracranial Haemorrhage 94
Next Generation Cerebo – Summative Usability Study of CEREBO in Traumatic Brain Injury Patients to Determine Ease of Use, Ease of Learning and Satisfaction 95
BioSerenity SAS Pipeline Products & Ongoing Clinical Trials Overview 96
MemoWave – Product Status 96
MemoWave – Product Description 96
BioSignal Analytics Inc Pipeline Products & Ongoing Clinical Trials Overview 97
AutoEEG – Product Status 97
AutoEEG – Product Description 97
Bio-Signal Group Corp. Pipeline Products & Ongoing Clinical Trials Overview 98
Wireless MicroEEG – Neonatal Apnea – Product Status 98
Wireless MicroEEG – Neonatal Apnea – Product Description 98
BioSignostix Inc. Pipeline Products & Ongoing Clinical Trials Overview 99
Neurosomex – Product Status 99
Neurosomex – Product Description 99
BiReme Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 100
Diagnostic Apparatus – Bipolar Disorder – Product Status 100
Diagnostic Apparatus – Bipolar Disorder – Product Description 100
Blackrock Microsystems LLC Pipeline Products & Ongoing Clinical Trials Overview 101
BrainGate2 Neural Interface System – Product Status 101
BrainGate2 Neural Interface System – Product Description 101
Blackrock Microsystems LLC – Ongoing Clinical Trials Overview 102
BrainGate2 Neural Interface System – A Sensorimotor Microelectrode Brain-machine Interface for Individuals with Tetraplegia 103
BrainGate2 Neural Interface System – Enhancement and Optimization of a Mobile iBCI for Veterans With Paralysis 103
BrainGate2 Neural Interface System – Feasibility Study of an Intracortical Neural Interface System for Persons with Tetraplegia: BrainGate2 103
BrainGate2 Neural Interface System – Single Neuron Population Dynamics in Human Speech Motor Cortex for a Speech Prosthesis 104
BrainGate2 Neural Interface System – Understanding and Restoring Speech Production Using an Intracortical Brain-computer Interface 104
Blinkcns Inc Pipeline Products & Ongoing Clinical Trials Overview 105
EyeStat – ADHD – Product Status 105
EyeStat – ADHD – Product Description 105
EyeStat – Migraine – Product Status 106
EyeStat – Migraine – Product Description 106
EyeStat – Traumatic Brain Injury (TBI) – Product Status 106
EyeStat – Traumatic Brain Injury (TBI) – Product Description 107
Blinktbi Inc Pipeline Products & Ongoing Clinical Trials Overview 108
EyeStat – Neurological Disease – Product Status 108
EyeStat – Neurological Disease – Product Description 108
Blue Iris Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 109
Circadian Stimulation Control Device – Product Status 109
Circadian Stimulation Control Device – Product Description 109
BMSEED LLC Pipeline Products & Ongoing Clinical Trials Overview 110
ECoG Electrode – Product Status 110
ECoG Electrode – Product Description 110
Boston Neurosciences Pipeline Products & Ongoing Clinical Trials Overview 111
Cerebral Autoregulation Device – Product Status 111
Cerebral Autoregulation Device – Product Description 111
Vittamed 105 – Product Status 112
Vittamed 105 – Product Description 112
Brain Scientific Inc Pipeline Products & Ongoing Clinical Trials Overview 113
Minimally Invasive Graphene Electrode – Epilepsy – Product Status 113
Minimally Invasive Graphene Electrode – Epilepsy – Product Description 113
Brain4Care Inc Pipeline Products & Ongoing Clinical Trials Overview 114
Brain4care Sensor – Wireless Version – Product Status 114
Brain4care Sensor – Wireless Version – Product Description 114
BrainCare OY Pipeline Products & Ongoing Clinical Trials Overview 115
UltimateEEG – Product Status 115
UltimateEEG – Product Description 115
Brainchem, LLC Pipeline Products & Ongoing Clinical Trials Overview 116
Intra-Operative Neuro-Monitoring Device – Epilepsy – Product Status 116
Intra-Operative Neuro-Monitoring Device – Epilepsy – Product Description 116
Intra-Operative Neuro-Monitoring Device – Traumatic Brain Injury – Product Status 117
Intra-Operative Neuro-Monitoring Device – Traumatic Brain Injury – Product Description 117
BrainScope Company Inc Pipeline Products & Ongoing Clinical Trials Overview 118
BrainScope Device – Alzheimer’s Disease – Product Status 118
BrainScope Device – Alzheimer’s Disease – Product Description 119
BrainScope Device – Depression – Product Status 119
BrainScope Device – Depression – Product Description 119
BrainScope Device – Seizure – Product Status 120
BrainScope Device – Seizure – Product Description 120
BrainScope Device – Stroke – Product Status 120
BrainScope Device – Stroke – Product Description 121
BrainScope Device – Teenage – Product Status 121
BrainScope Device – Teenage – Product Description 121
BrainScope NT-1000 – Product Status 122
BrainScope NT-1000 – Product Description 122
BrainScope One – Pediatric – Product Status 122
BrainScope One – Pediatric – Product Description 123
Braintech Ltd. Pipeline Products & Ongoing Clinical Trials Overview 124
BrainTech Mental Disorders Diagnosis – Product Status 124
BrainTech Mental Disorders Diagnosis – Product Description 124
Brazen Pipeline Products & Ongoing Clinical Trials Overview 125
Brazen Device – Product Status 125
Brazen Device – Product Description 125
Byteflies Pipeline Products & Ongoing Clinical Trials Overview 126
Byteflies Kit – Acute Viral Infections – Product Status 126
Byteflies Kit – Acute Viral Infections – Product Description 126
Byteflies Kit – Neurodegenerative Disease – Product Status 127
Byteflies Kit – Neurodegenerative Disease – Product Description 127
Byteflies Kit – Sleep Disorders – Product Status 127
Byteflies Kit – Sleep Disorders – Product Description 128
EpiCare@Home – Product Status 128
EpiCare@Home – Product Description 129
Byteflies – Ongoing Clinical Trials Overview 130
EpiCare@Home – Validation Study for Monitoring of Focal Onset Epileptic Seizures With a Wearable Seizure Monitoring Device, EpiCare@Home 131
C. Light Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 132
TSLO – ALS – Product Status 132
TSLO – ALS – Product Description 132
TSLO – Alzheimer’s Disease – Product Status 133
TSLO – Alzheimer’s Disease – Product Description 133
TSLO – Concussions – Product Status 133
TSLO – Concussions – Product Description 134
TSLO – Multiple Sclerosis – Product Status 134
TSLO – Multiple Sclerosis – Product Description 134
TSLO – Parkinson’s Disease – Product Status 135
TSLO – Parkinson’s Disease – Product Description 135
California Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 136
Digital TRUE – Product Status 136
Digital TRUE – Product Description 136
CEA-Leti Pipeline Products & Ongoing Clinical Trials Overview 137
Disruptive Magnetoencephalography System – Product Status 137
Disruptive Magnetoencephalography System – Product Description 137
Cephalogics, LLC Pipeline Products & Ongoing Clinical Trials Overview 138
High-Density Diffuse Optical Tomography System – Product Status 138
High-Density Diffuse Optical Tomography System – Product Description 138
CergenX Ltd Pipeline Products & Ongoing Clinical Trials Overview 139
Newborn Brain Screener – Product Status 139
Newborn Brain Screener – Product Description 139
Ceribell Inc Pipeline Products & Ongoing Clinical Trials Overview 140
Ceribell Pocket EEG Device – Delirium – Product Status 140
Ceribell Pocket EEG Device – Delirium – Product Description 140
Cerora Inc Pipeline Products & Ongoing Clinical Trials Overview 141
Cerora Borealis – Product Status 141
Cerora Borealis – Product Description 141
MindReader – Product Status 142
MindReader – Product Description 142
MindReader – Autism – Product Status 142
MindReader – Autism – Product Description 143
MindReader – Neurodegenerative Conditions – Product Status 143
MindReader – Neurodegenerative Conditions – Product Description 144
Champaign Imaging LLC Pipeline Products & Ongoing Clinical Trials Overview 145
Next Generation Functional Neuroimaging Tool – Product Status 145
Next Generation Functional Neuroimaging Tool – Product Description 145
Next Generation Pediatric Neuroimaging Tool – Product Status 146
Next Generation Pediatric Neuroimaging Tool – Product Description 146
Children’s Hospital of Philadelphia Pipeline Products & Ongoing Clinical Trials Overview 147
Hand-Held DIP – Concussion – Product Status 147
Hand-Held DIP – Concussion – Product Description 147
Cimon Medical AS Pipeline Products & Ongoing Clinical Trials Overview 148
NeoDoppler – Product Status 148
NeoDoppler – Product Description 148
Cincinnati Children’s Hospital Medical Center Pipeline Products & Ongoing Clinical Trials Overview 149
Diagnostic System – Concussion – Product Status 149
Diagnostic System – Concussion – Product Description 149
Circadian Therapeutics Pipeline Products & Ongoing Clinical Trials Overview 150
Circaid EEG – Product Status 150
Circaid EEG – Product Description 150
City, University of London Pipeline Products & Ongoing Clinical Trials Overview 151
Optical Fibre System – Product Status 151
Optical Fibre System – Product Description 151
ClearPoint Neuro Inc Pipeline Products & Ongoing Clinical Trials Overview 152
Microelectric Recording (MER) System – Product Status 152
Microelectric Recording (MER) System – Product Description 152
ClearSky Medical Diagnostics Ltd Pipeline Products & Ongoing Clinical Trials Overview 153
MCI-Monitor – Product Status 153
MCI-Monitor – Product Description 153
Cleveland Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 154
Electroencephalogram Electrode – Product Status 154
Electroencephalogram Electrode – Product Description 154
Cognionics, Inc. Pipeline Products & Ongoing Clinical Trials Overview 155
EEG Headset System – Product Status 155
EEG Headset System – Product Description 155
Stress/Strain Monitoring Device – Product Status 156
Stress/Strain Monitoring Device – Product Description 156
Cognitive Sensing Inc. Pipeline Products & Ongoing Clinical Trials Overview 157
NeuroMinder MPI Early Detection Device – Product Status 157
NeuroMinder MPI Early Detection Device – Product Description 157
Cogwear LLC Pipeline Products & Ongoing Clinical Trials Overview 158
EEG Device – Product Status 158
EEG Device – Product Description 158
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 159
ExtraVentricular Drain – Product Status 159
ExtraVentricular Drain – Product Description 159
Columbia University Medical Center Pipeline Products & Ongoing Clinical Trials Overview 160
Online Intracranial EEG Acquisition System – Product Status 160
Online Intracranial EEG Acquisition System – Product Description 160
Compumedics Alpha Trace GmbH Pipeline Products & Ongoing Clinical Trials Overview 161
Diagnostic Tool – Alzheimer’s Disease – Product Status 161
Diagnostic Tool – Alzheimer’s Disease – Product Description 161
Diagnostic Tool – Dementia – Product Status 162
Diagnostic Tool – Dementia – Product Description 162
Compumedics Germany GmbH Pipeline Products & Ongoing Clinical Trials Overview 163
DWL TBI System – Product Status 163
DWL TBI System – Product Description 163
Compumedics Ltd Pipeline Products & Ongoing Clinical Trials Overview 164
Long Term Epilepsy Monitoring Device – Product Status 164
Long Term Epilepsy Monitoring Device – Product Description 164
Converge Medical, Inc Pipeline Products & Ongoing Clinical Trials Overview 165
Brain Function Monitor – Product Status 165
Brain Function Monitor – Product Description 165
CranioSense LLC Pipeline Products & Ongoing Clinical Trials Overview 166
Vivonics IPASS – Product Status 166
Vivonics IPASS – Product Description 166
CREmedical Corp Pipeline Products & Ongoing Clinical Trials Overview 167
Tripolar Concentric Ring Electrode (TCRE) Sensor – Product Status 167
Tripolar Concentric Ring Electrode (TCRE) Sensor – Product Description 167
CREmedical Corp – Ongoing Clinical Trials Overview 168
Tripolar Concentric Ring Electrode (TCRE) Sensor – Epilepsy Seizure Detection With Innovative Tripolar EEG (tEEG) 169
CyberneX Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 170
Brain Computer Interface Device – Product Status 170
Brain Computer Interface Device – Product Description 170
Dartmouth College Pipeline Products & Ongoing Clinical Trials Overview 171
Analog-To-Digital Converter (ADC) Device – Product Status 171
Analog-To-Digital Converter (ADC) Device – Product Description 171
Optode With Cap System – Product Status 172
Optode With Cap System – Product Description 172
DMetrix Inc Pipeline Products & Ongoing Clinical Trials Overview 173
V6 – Product Status 173
V6 – Product Description 173
Drexel University Pipeline Products & Ongoing Clinical Trials Overview 174
Brain Edema Monitoring System – Product Status 174
Brain Edema Monitoring System – Product Description 174
BRAin State RECognition System – Product Status 175
BRAin State RECognition System – Product Description 175
Microwave Wireless Implant – Product Status 175
Microwave Wireless Implant – Product Description 176
Duke University Pipeline Products & Ongoing Clinical Trials Overview 177
Passive Pressure Sensor – Product Status 177
Passive Pressure Sensor – Product Description 177
Ecole Polytechnique Federale de Lausanne Pipeline Products & Ongoing Clinical Trials Overview 178
Brain Fingerprint – Mild Cognitive Impairment – Product Status 178
Brain Fingerprint – Mild Cognitive Impairment – Product Description 178
EIC Laboratories, Inc. Pipeline Products & Ongoing Clinical Trials Overview 179
Wireless EcoG Recording System – Epilepsy – Product Status 179
Wireless EcoG Recording System – Epilepsy – Product Description 179
Electrical Geodesics Inc Pipeline Products & Ongoing Clinical Trials Overview 180
Brain Bleeding Monitor – Product Status 180
Brain Bleeding Monitor – Product Description 180
dEEG/NIRS System – Infants – Product Status 181
dEEG/NIRS System – Infants – Product Description 181
EEG Ink Net – Product Status 181
EEG Ink Net – Product Description 182
ElMinda Ltd Pipeline Products & Ongoing Clinical Trials Overview 183
BNA Analysis System – ADHD – Product Status 183
BNA Analysis System – ADHD – Product Description 183
BNA Analysis System – Alzheimer’s Disease – Product Status 184
BNA Analysis System – Alzheimer’s Disease – Product Description 184
BNA Analysis System – Migraine – Product Status 184
BNA Analysis System – Migraine – Product Description 185
BNA Analysis System – Mood Disorder – Product Status 185
BNA Analysis System – Mood Disorder – Product Description 185
BNA Analysis System – Pain – Product Status 186
BNA Analysis System – Pain – Product Description 186
BNA Analysis System – Parkinson’s Disease – Product Status 186
BNA Analysis System – Parkinson’s Disease – Product Description 187
Embryyo Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 188
Hydrocephalus Monitoring Device – Product Status 188
Hydrocephalus Monitoring Device – Product Description 188
Emosis Pte Ltd Pipeline Products & Ongoing Clinical Trials Overview 189
Emosis Headband – Product Status 189
Emosis Headband – Product Description 189
EMTensor Gmbh Pipeline Products & Ongoing Clinical Trials Overview 190
4 ET Brain Scanning Device – Product Status 190
4 ET Brain Scanning Device – Product Description 190
EMTensor Brain Scanner – Ambulance – Product Status 191
EMTensor Brain Scanner – Ambulance – Product Description 191
EMTensor Brain Scanner – Bedside – Product Status 191
EMTensor Brain Scanner – Bedside – Product Description 192
EMTensor Brain Scanner – Continuous Monitoring – Product Status 192
EMTensor Brain Scanner – Continuous Monitoring – Product Description 192
Epi-Minder Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 193
Minder – Product Status 193
Minder – Product Description 193
Epi-Minder Pty Ltd – Ongoing Clinical Trials Overview 194
Minder – A Prospective Study to Assess the Safety of a Sub-scalp Monitoring Device for the Recording of Brain Electrical Activity Associated with the Occurrence of Epileptic Seizures 195
Epitel, Inc. Pipeline Products & Ongoing Clinical Trials Overview 196
Aura System – Product Status 196
Aura System – Product Description 196
Evon Medics LLC Pipeline Products & Ongoing Clinical Trials Overview 197
EMRAST – Product Status 197
EMRAST – Product Description 197
Ewear Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 198
EEG Sensor – Product Status 198
EEG Sensor – Product Description 198
Eyefluence Corp Pipeline Products & Ongoing Clinical Trials Overview 199
Eye-Com Biosensor – Product Status 199
Eye-Com Biosensor – Product Description 199
FieldLine Inc Pipeline Products & Ongoing Clinical Trials Overview 200
Micro-OPM Wearable Full-Head MEG System – Product Status 200
Micro-OPM Wearable Full-Head MEG System – Product Description 200
On-Scalp MEG Device – Product Status 201
On-Scalp MEG Device – Product Description 201
Flint Hills Scientific LLC Pipeline Products & Ongoing Clinical Trials Overview 202
Seizure Detection Device – Product Status 202
Seizure Detection Device – Product Description 202
Florida International University Pipeline Products & Ongoing Clinical Trials Overview 203
Tremor Detection Device – Product Status 203
Tremor Detection Device – Product Description 203
Forest Devices Pipeline Products & Ongoing Clinical Trials Overview 204
AlphaStroke – Product Status 204
AlphaStroke – Product Description 204
Forest Devices – Ongoing Clinical Trials Overview 205
AlphaStroke – Emergency Data Gathering and AlphaStroke Refinement: EDGAR 206
Formsense Pipeline Products & Ongoing Clinical Trials Overview 207
mHealth System – Product Status 207
mHealth System – Product Description 207
Futurecure Health Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 208
Diagnostic Device – Dizziness – Product Status 208
Diagnostic Device – Dizziness – Product Description 208
Diagnostic Device – Migraine – Product Status 209
Diagnostic Device – Migraine – Product Description 209
g.tec medical engineering GmbH Pipeline Products & Ongoing Clinical Trials Overview 210
mindBEAGLE – Product Status 210
mindBEAGLE – Product Description 210
Georgetown University Pipeline Products & Ongoing Clinical Trials Overview 211
Subdural Electrocorticography Device – Product Status 211
Subdural Electrocorticography Device – Product Description 211
Georgia Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 212
Brain Buddy – Product Status 212
Brain Buddy – Product Description 212
Wireless Neurovascular Monitoring System – Product Status 213
Wireless Neurovascular Monitoring System – Product Description 213
Haystack Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 214
Needle iEMG Device – Product Status 214
Needle iEMG Device – Product Description 214
HeadSense Medical Ltd. Pipeline Products & Ongoing Clinical Trials Overview 215
HeadSense – Cerebral Vasospasm – Product Status 215
HeadSense – Cerebral Vasospasm – Product Description 215
HS-1000 – Concussion – Product Status 216
HS-1000 – Concussion – Product Description 216
HEARD Medical Pipeline Products & Ongoing Clinical Trials Overview 217
Dry EEG Sensor – Bioamplifer System – Product Status 217
Dry EEG Sensor – Bioamplifer System – Product Description 217
Hebrew University of Jerusalem Pipeline Products & Ongoing Clinical Trials Overview 218
Brainwatch – Continuous Pupil Monitoring – Product Status 218
Brainwatch – Continuous Pupil Monitoring – Product Description 218
Highland Instruments Inc Pipeline Products & Ongoing Clinical Trials Overview 219
EEG Device – Opioid Use Disorder – Product Status 219
EEG Device – Opioid Use Disorder – Product Description 219
Hong Kong Polytechnic University Pipeline Products & Ongoing Clinical Trials Overview 220
Intelligent System – Ischemic Stroke – Product Status 220
Intelligent System – Ischemic Stroke – Product Description 220
iBam Technologies Pipeline Products & Ongoing Clinical Trials Overview 221
indirect Brainstem Activity Monitor – Product Status 221
indirect Brainstem Activity Monitor – Product Description 221
IMEC Pipeline Products & Ongoing Clinical Trials Overview 222
Eight Channel Wireless EEG Headset – Product Status 222
Eight Channel Wireless EEG Headset – Product Description 222
iMediSync Inc Pipeline Products & Ongoing Clinical Trials Overview 223
iSyncWave – Product Status 223
iSyncWave – Product Description 223
Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 224
iPROBE – Product Status 224
iPROBE – Product Description 224
Indian Institute of Technology Bombay Pipeline Products & Ongoing Clinical Trials Overview 225
Electro Diagnostic Digital Instrument – Product Status 225
Electro Diagnostic Digital Instrument – Product Description 225
Indian Institute of Technology Delhi Pipeline Products & Ongoing Clinical Trials Overview 226
Diagnostic Device – Epilepsy – Product Status 226
Diagnostic Device – Epilepsy – Product Description 226
Infinite Biomedical Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 227
Neonatal Neurological Monitor (N2M) – Product Status 227
Neonatal Neurological Monitor (N2M) – Product Description 227
InfraScan Inc Pipeline Products & Ongoing Clinical Trials Overview 228
Infrascanner Model 2000 – Children – Product Status 228
Infrascanner Model 2000 – Children – Product Description 228
Next -Generation Infrascanner Brain Hematoma Detector – Product Status 229
Next -Generation Infrascanner Brain Hematoma Detector – Product Description 229
InfraScan Inc – Ongoing Clinical Trials Overview 230
Infrascanner Model 2000 – Children – Defining the Operating Characteristics of Near-Infrared Spectroscopy (NIRS) in The Diagnosis of Pediatric Traumatic Intracranial Hemorrhage 231
Innovative Biomedical Instruments and Systems Pipeline Products & Ongoing Clinical Trials Overview 232
Electroencephelograph System – Product Status 232
Electroencephelograph System – Product Description 232
Instituto de Medicina Genomica SL Pipeline Products & Ongoing Clinical Trials Overview 233
Diagnostic Tool – Neurotoxicity – Product Status 233
Diagnostic Tool – Neurotoxicity – Product Description 233
Integer Holdings Corp Pipeline Products & Ongoing Clinical Trials Overview 234
Deep Brain Mapping Micro-Electrode – Product Status 234
Deep Brain Mapping Micro-Electrode – Product Description 234
Electrocorticography Micro-electrode – Product Status 235
Electrocorticography Micro-electrode – Product Description 235
Integra LifeSciences Holdings Corp Pipeline Products & Ongoing Clinical Trials Overview 236
Brain Mapping Device – Product Status 236
Brain Mapping Device – Product Description 236
Critical Care Neuro Monitoring System – Product Status 237
Critical Care Neuro Monitoring System – Product Description 237
Integrated Sensing Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 238
Wireless Intracranial Pressure Monitor – Product Status 238
Wireless Intracranial Pressure Monitor – Product Description 239
Irras AB Pipeline Products & Ongoing Clinical Trials Overview 240
Hummingbird ICP Control Module – Product Status 240
Hummingbird ICP Control Module – Product Description 241
Hummingbird Quad ICP Monitoring with Drainage – Product Status 241
Hummingbird Quad ICP Monitoring with Drainage – Product Description 241
Hummingbird Solo ICP Monitoring – Product Status 242
Hummingbird Solo ICP Monitoring – Product Description 242
Hummingbird Subdural H900DS (Drainage And ICP) – Product Status 242
Hummingbird Subdural H900DS (Drainage And ICP) – Product Description 243
IRRAflow – Chronic Subdural Hematomas – Product Status 243
IRRAflow – Chronic Subdural Hematomas – Product Description 243
IRRAflow 3.0 – Product Status 244
IRRAflow 3.0 – Product Description 244
IRRAflow CNS System – Product Status 245
IRRAflow CNS System – Product Description 245
Irras AB – Ongoing Clinical Trials Overview 246
IRRAflow CNS System – Active Removal of IntraCerebral Hematoma Via Active Irrigation of the Ventricular System 247
IRRAflow CNS System – DIVE: Deployment of Irrigating Intraventricular Catheter System for Intraventricular Hemorrhage 247
IRRAflow CNS System – Study Evaluating the Effectiveness of IRRAflow System in the Treatment of Patients Across Multiple Intracranial Pathologies 247
IRRAflow CNS System – Use of Active Fluid Exchange to Therapeutically Treat Intracranial Bleeding and Infection 248
IRRAflow CNS System – Use of ACTIVE Fluid Exchange to Treat Intraventricular Hemorrhage 248
IScreen 2 Prevent LLC Pipeline Products & Ongoing Clinical Trials Overview 249
Neurotechnology Device – Alzheimer’s Disease – Product Status 249
Neurotechnology Device – Alzheimer’s Disease – Product Description 249
Jan Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 250
BrainPulse 1100 – Concussion – Product Status 250
BrainPulse 1100 – Concussion – Product Description 250
Jan Medical, Inc. – Ongoing Clinical Trials Overview 251
BrainPulse 1100 – Concussion – Non-blinded Data Collection Pilot Study of Acute Stroke Using the Brainpulse 252
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 253
Brain Sensing Device – Product Status 253
Brain Sensing Device – Product Description 253
Photoacoustic Imaging – Ischemic Stroke – Product Status 254
Photoacoustic Imaging – Ischemic Stroke – Product Description 254
Sonolucent Intracranial Pressure Monitor – Product Status 254
Sonolucent Intracranial Pressure Monitor – Product Description 255
Transcranial Ultrasound Lens – Product Status 255
Transcranial Ultrasound Lens – Product Description 255
KeepAlive Medical, Ltd. Pipeline Products & Ongoing Clinical Trials Overview 256
Dementia Monitor – Product Status 256
Dementia Monitor – Product Description 256
Kernel Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 257
Kernel Flow Device – Product Status 257
Kernel Flow Device – Product Description 258
Kernel Inc (Inactive) – Ongoing Clinical Trials Overview 259
Kernel Flow Device – A Randomized Study Comparing the Effect of RelieVRx VR Based Program and VR Program on the Brains of Patients with Chronic Pain 260
Kernel Flow Device – IMPACT: Investigating Mild Cognitive Impairment in Patients And Controls With TD-fNIRS 260
Klick Health Pipeline Products & Ongoing Clinical Trials Overview 261
SymPulse Tele-Empathy Device – Product Status 261
SymPulse Tele-Empathy Device – Product Description 261
Koronis Biomedical Technologies Corporation Pipeline Products & Ongoing Clinical Trials Overview 262
Non-Invasive Intracranial Pressure Monitor – Product Status 262
Non-Invasive Intracranial Pressure Monitor – Product Description 262
Non-Invasive Tool – Cognition Assessment – Product Status 263
Non-Invasive Tool – Cognition Assessment – Product Description 263
Korwave Pipeline Products & Ongoing Clinical Trials Overview 264
Korwave – Product Status 264
Korwave – Product Description 264
Kriyaneuro Technologies Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 265
Wireless Limb Band – Parkinson’s Disease – Product Status 265
Wireless Limb Band – Parkinson’s Disease – Product Description 265
Kumamoto University Pipeline Products & Ongoing Clinical Trials Overview 266
Seizure Prediction Device – Product Status 266
Seizure Prediction Device – Product Description 266
Lara Diagnostics AB Pipeline Products & Ongoing Clinical Trials Overview 267
Nerve Damage Diagnosis Instrument – Product Status 267
Nerve Damage Diagnosis Instrument – Product Description 267
Leap Medical Inc. (Inactive) Pipeline Products & Ongoing Cli
![]()