Non-Invasive Prenatal Testing (NIPT) Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Non-invasive prenatal screening is a method that determines the risk of genetic abnormalities in the fetus. The Non-invasive prenatal testing (NIPT) pipeline market research report provides comprehensive information about the Non-invasive prenatal testing (NIPT) pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
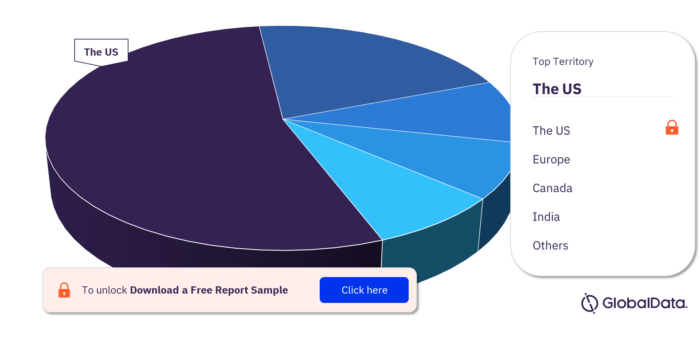
Territory Outlook of the NIPT Pipeline Products Market
The US, Europe, Canada, India, Singapore, and the UK have products in the NIPT pipeline product market. As of July 2022, the US has the highest number of products in the pipeline out of them all.
NIPT Pipeline Products Market, by Territory
For more territory insights, download a free report sample
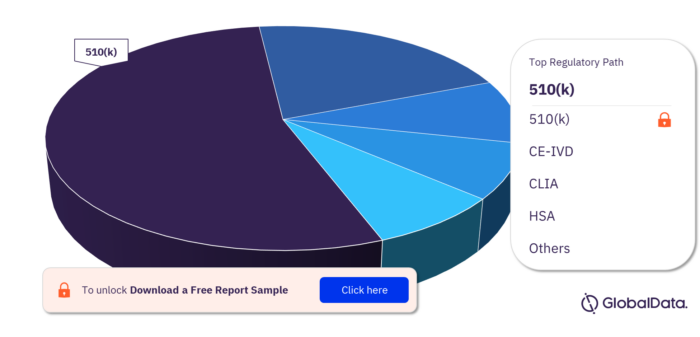
Key Regulatory Paths for the NIPT Pipeline Products Market
The regulatory paths followed by the NIPT pipeline products are 510(k), CE-IVD, CLIA, HSA, ICAC, MDL, PMA, and UKCA. Most of the products follow the 510(k) pathway to enter the market.
NIPT Pipeline Products Market, by Regulatory Path
For more regulatory path insights, download a free report sample
Key Companies in the NIPT Pipeline Products Market
Some of the key companies in the NIPT pipeline products market are Agena Bioscience Inc, Amity University, BCML GmbH, BioCeps (Inactive), Biocept Inc, Biofidelity Ltd, BioFluidica Microtechnologies LLC, Cardiff Oncology Inc, Celula Inc, and ChromaCode Inc.
Agena Bioscience Inc
Agena Bioscience Inc operates as a medical device company. The company products are used for genotyping and mutation detection, ultrasensitive detection, methylation analysis, oncology, and other applications. Agena Bioscience is headquartered in San Diego, California, the US.
Amity University
Amity University is an educational institution that undertakes various national and international research activities. The institution has global campuses in London, Dubai, New York, Singapore, Mauritius, Seattle, Abu Dhabi, South Africa, San Francisco, Romania, China, and Amsterdam. Amity is headquartered in Noida, India.
Biocept Inc
Biocept Inc is a molecular diagnostics company. They develop and commercialize proprietary circulating tumor cell (CTC) and circulating tumor DNA assays utilizing a standard blood sample. They also serve cancer diagnostic assays to oncologists and other physicians, hospitals, and cancer centers. Biocept is headquartered in San Diego, California, the US.
Biofidelity Ltd
Biofidelity Ltd. is a molecular assay company that develops molecular diagnostic assay that enables the rapid detection of target DNA sequences. The company is headquartered in the UK.
Cardiff Oncology Inc
Cardiff Oncology Inc is a clinical-stage biotechnology company that focuses on developing drugs that target cell division using precision cancer medicine (PCM) approach for the treatment of hematologic and solid tumor cancers. Cardiff Oncology is headquartered in San Diego, California, the US.
Market Report Overview
| Key Territories | The US, Europe, Canada, India, Singapore, and The UK |
| Key Regulatory Paths | 510(k), CE-IVD, CLIA, HSA, ICAC, MDL, PMA, and UKCA |
| Key Companies | Agena Bioscience Inc, Amity University, BCML GmbH, BioCeps (Inactive), Biocept Inc, Biofidelity Ltd, BioFluidica Microtechnologies LLC, Cardiff Oncology Inc, Celula Inc, and ChromaCode Inc |
Scope
This report provides:
- Extensive coverage of the Non-Invasive Prenatal Testing (NIPT) under development.
- Details of major pipeline products which include product description, licensing, collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Non-Invasive Prenatal Testing (NIPT) and lists all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with a potentially strong product portfolio and create effective counterstrategies to gain a competitive advantage.
- Identify and understand important and diverse types of Non-Invasive Prenatal Testing (NIPT) under development.
- Develop market entry and market expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Amity University
BCML GmbH
BioCeps (Inactive)
Biocept Inc
Biofidelity Ltd
BioFluidica Microtechnologies LLC
Cardiff Oncology Inc
Celula Inc
ChromaCode Inc
Echelon Diagnostics Inc
Elysium Health Inc
Fluxion Biosciences Inc
Helicos BioSciences Corporation
Illumina Inc
INEX Innovate Pte Ltd
Johns Hopkins University
Juno Diagnostics Inc
KellBenx Inc (Inactive)
Laval University
Maastricht University Medical Center
Natera Inc
Oxford Gene Technology Ltd
PerkinElmer Inc
Sequenom Inc
Standard BioTools Inc
StellarGene Technologies Pvt Ltd
The Chaim Sheba Medical Center
The Chinese University of Hong Kong
thromboDx BV
Tufts Medical Center
University of Oxford
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key territories in the NIPT pipeline products market?
The US, Europe, Canada, India, Singapore, and the UK have products in the pipeline.
-
What are the key regulatory paths for the NIPT pipeline products market?
The key regulatory paths followed by the NIPT pipeline products are 510(k), CE-IVD, CLIA, HSA, ICAC, MDL, PMA, and UKCA.
-
What are the key companies in the NIPT pipeline products market?
Some of the key companies in the NIPT pipeline products market are Agena Bioscience Inc, Amity University, BCML GmbH, BioCeps (Inactive), Biocept Inc, Biofidelity Ltd, BioFluidica Microtechnologies LLC, Cardiff Oncology Inc, Celula Inc, and ChromaCode Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more In Vitro Diagnostics reports