Orthotic Devices Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Orthotic Devices Pipeline Market Report Overview
Orthotic Devices are used for supporting an injured part in both pre- and post-operative procedures. The orthotic devices pipeline market research report provides a comprehensive understanding of the pipeline products and their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Segments | · Orthopedic Braces and Supports
· Upper Extremity Braces and Supports · Ankle & Foot Braces and Supports · Knee Braces and Supports · Spinal Braces and Supports |
| Key Territories | · The US
· Europe · India · Israel · Japan |
| Key Regulatory Paths | · 510(k)
· CE · ICAC · MDL · Ninsho |
| Leading Companies | · AbiliTech Medical Inc
· AidPlex · AIM Laboratory · Biomotum Inc · Bionic Power Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Orthotic Devices Pipeline Market by Segments
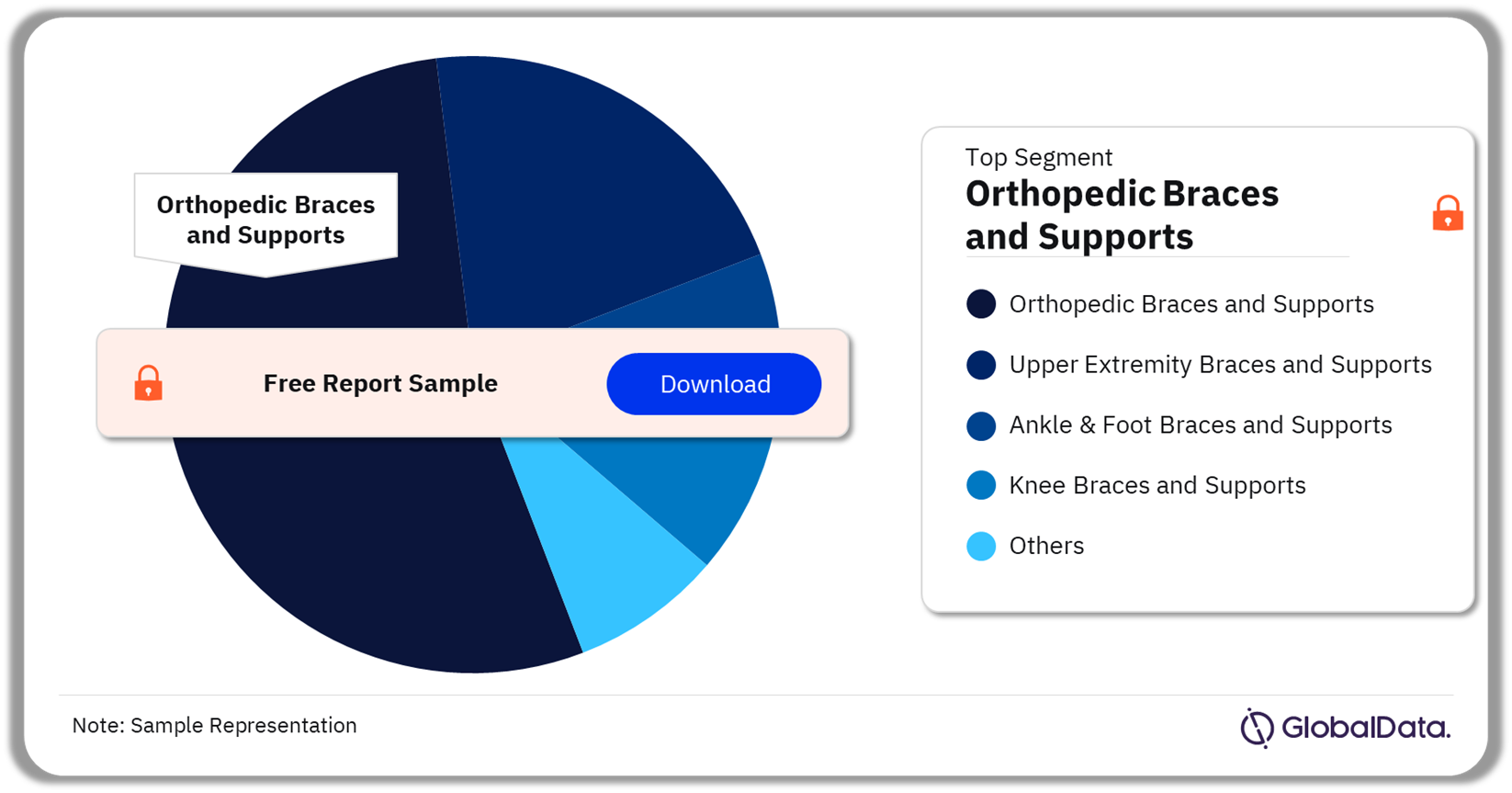
The key segments in the orthotic devices pipeline market are orthopedic braces and supports, upper extremity braces and supports, ankle & foot braces and supports, knee braces and supports, and spinal braces and supports among others. As of December 2023, orthopedic braces and supports were the leading segment accounting for the highest number of products in the pipeline.
Orthotic Devices Pipeline Market Analysis by Segments, 2023 (%)
Buy the Full Report for More Segment Insights into the Orthotic Devices Pipeline Market
Orthotic Devices Pipeline Market Segmentation by Territories
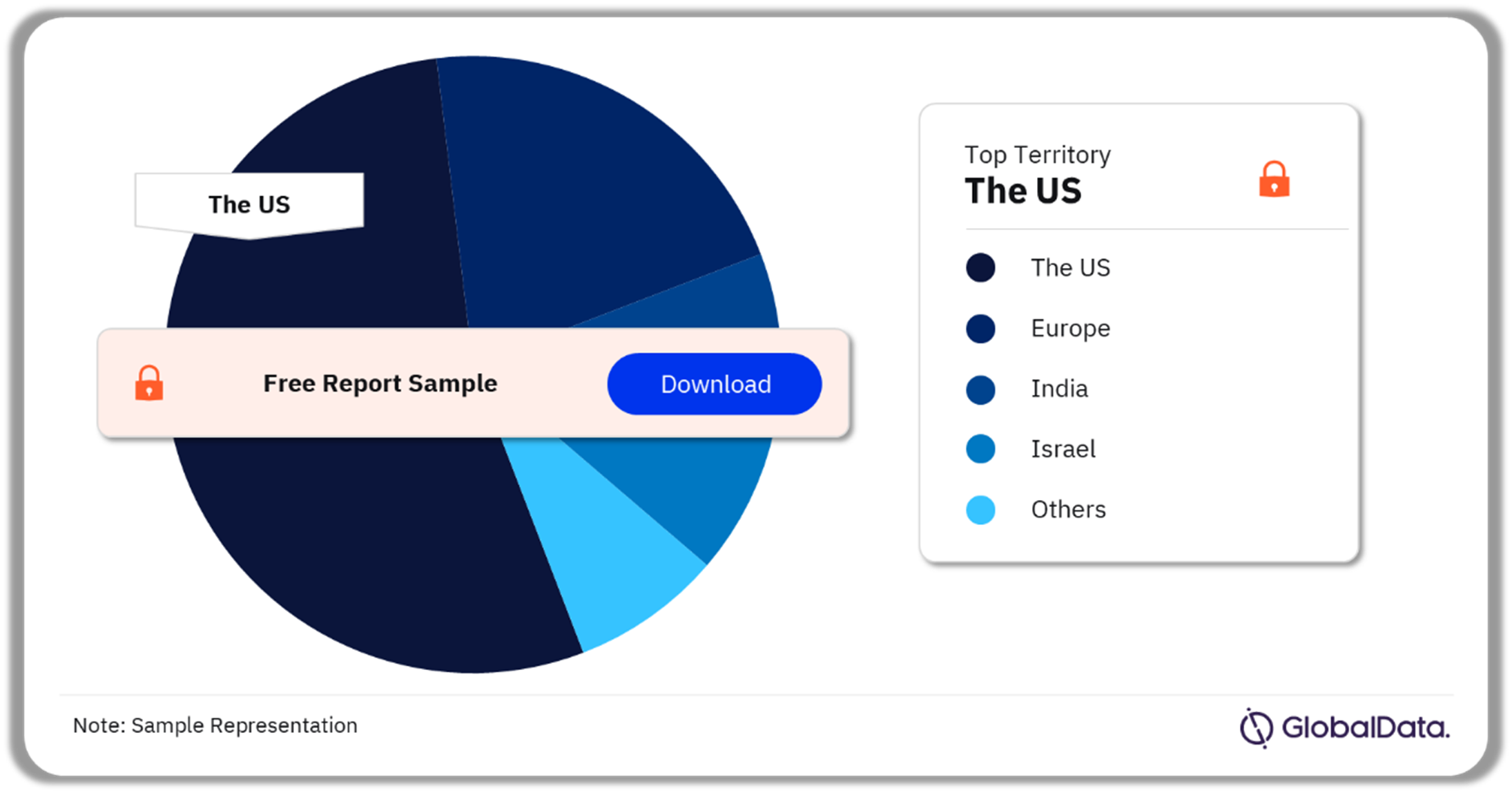
A few of the key territories for orthotic devices pipeline products are the US, Europe, Canada, India, Israel, and Japan among others. As of December 2023, the US accounted for the highest number of pipeline products.
Orthotic Devices Pipeline Market Analysis by Territories, 2023 (%)
Buy the Full Report for More Territory Insights into the Orthotic Devices Pipeline Market
Orthotic Devices Pipeline Market Segmentation by Regulatory Paths
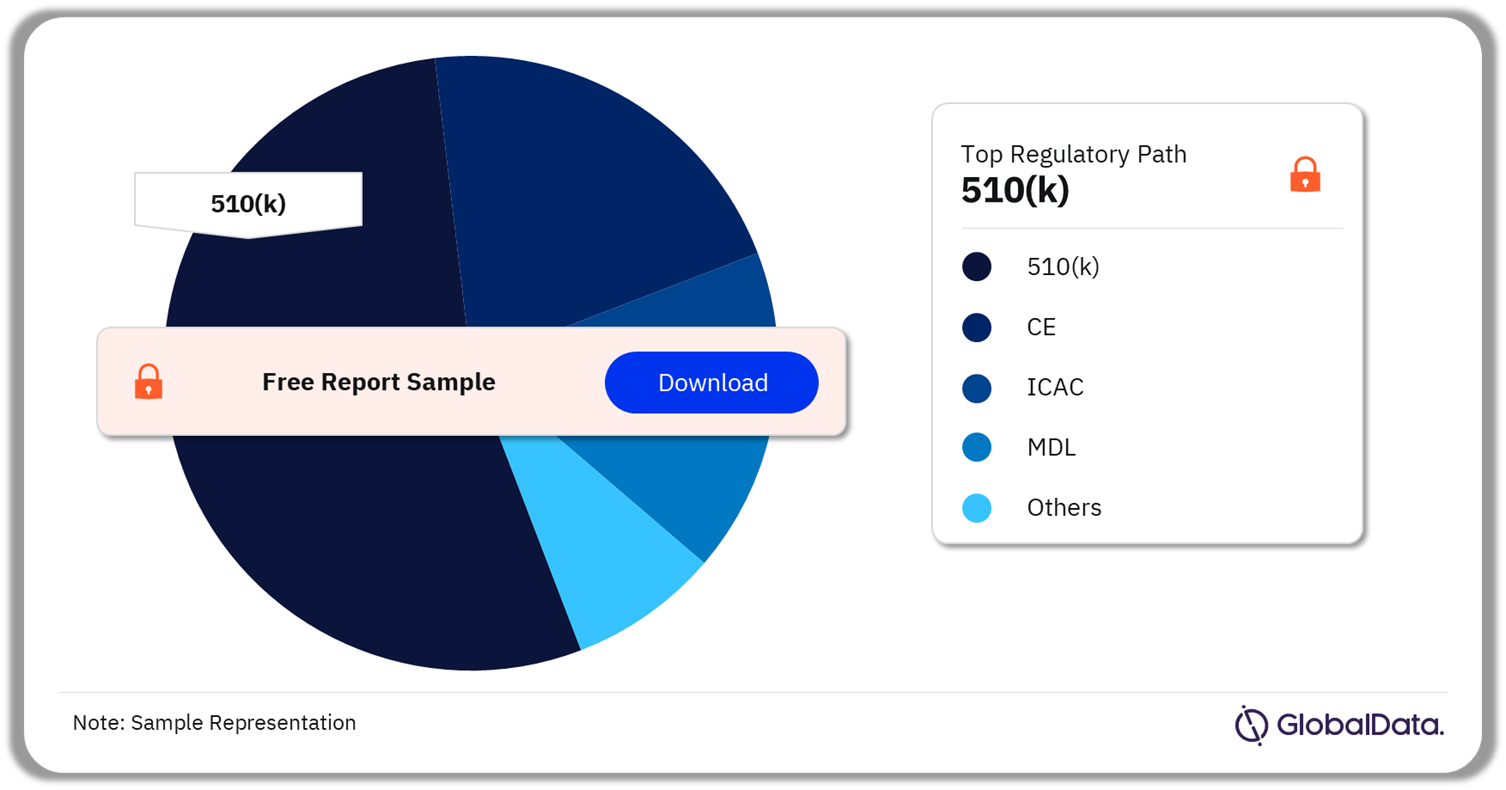
A few of the key regulatory paths followed by the orthotic devices pipeline market are 510(k), CE, ICAC, MDL, and Ninsho. As of December 2023, 510(k) was the most followed pathway for pipeline products in the market.
Orthotic Devices Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy the Full Report for More Regulatory Path Insights into the Orthotic Devices Pipeline Market
Orthotic Devices Pipeline Market – Competitive Landscape
A few of the key companies associated with the orthotic devices pipeline market are AbiliTech Medical Inc, AidPlex, AIM Laboratory, Biomotum Inc, and Bionic Power Inc., among others.
AbiliTech Medical Inc: AbiliTech is headquartered in Saint Paul, Minnesota, the US. The company is a manufacturer and supplier of medical devices which provides functional assistance and support for neuromuscular disorders. The company product includes AbiliTech Assist that provides functional assistance and support to the elbow and shoulder. It serves in therapeutic areas of neuromuscular weakness. The company offers its services to clinical partners, patients, and clinicians.
Biomotum Inc: Biomotum is headquartered in Portland, Oregon, the US. Biomotum Inc (Biomotum) is a medical manufacturing company that focuses on optimizing human mobility by providing intelligent and intuitive wearable systems and offering its users in the medical and consumer markets.
Orthotic Devices Pipeline Market Analysis by Companies, 2023
Buy the Full Report for More Company Insights into the Orthotic Devices Pipeline Market
Download a Free Report Sample
Segments Covered in the Report
Orthotic Devices Pipeline Market Segments Outlook
- Orthopedic Braces and Supports
- Upper Extremity Braces and Supports
- Ankle & Foot Braces and Supports
- Knee Braces and Supports
- Spinal Braces and Supports
Orthotic Devices Pipeline Market Territories Outlook
- The US
- Europe
- Canada
- India
- Israel
Orthotic Devices Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- ICAC
- MDL
- Ninsho
Scope
This report provides:
- Extensive coverage of the orthotic devices under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of orthotic devices and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of orthotic devices under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
AidPlex
AIM Laboratory
Baltimore VA Medical Center
Bio-Mechanical Composites Inc
Biomotum Inc
Bionic Power Inc
Brandeis University
Cambridge University Hospitals NHS Foundation Trust
Clear Advantage Collar, Inc. (Inactive)
Columbia University
Columbia University Medical Center
Cyberdyne Inc
Drexel University
Enovis Corp
Exo Dynamics, LLC
Fraunhofer-Gesellschaft zur Forderung der Angewandten Forschung eV
Georgia Institute of Technology
Green Sun Medical
Hansjorg Wyss Institute for Biologically Inspired Engineering
Holey Srl
Indian Institute of Technology Bombay
Indian Institute of Technology Delhi
Koc University
Kumudha Health Tech Pvt Ltd
Liberating Technologies Inc
Louis Stokes Cleveland VA Medical Center
Manchester University NHS Foundation Trust
Manometric BV
Maxa Bracing LLC
MediAsha Technologies Pvt Ltd
Medical University of South Carolina
MediCarbone Inc
Minneapolis VA Medical Center
National University of Singapore
Newndra Innovations Pvt Ltd
North Carolina State University
Northern Illinois University
Orthoflex Ltd
Orthopedic Institute PC
Ossur hf
Ottawa Hospital Research Institute
Oxford University Innovation Ltd
Polytechnic University of Catalonia
Redyns Medical LLC
Reev SAS
Resultados y Calidad del Sistema Sanitario Publico de Andalucia
Rice University
Stanford University
The Hospital for Sick Children
Tynor Orthotics Pvt Ltd
U.S. Department of Defence
University Hospital Hradec Kralove
University of Antwerp
University of Arizona
University of Bristol
University of California San Diego
University of Canterbury
University of Colorado
University of Delaware
University of Denver
University of Florida
University of Glasgow
University of Melbourne
University of Missouri
University of North Carolina
University of South Florida
University of Technology Sydney
University of Texas Southwestern Medical Center
University of Wisconsin-Stout
UNYQ Design Inc
Vanderbilt University
Victoria University
Wellinks Inc
Table of Contents
Table
Figures
Frequently asked questions
-
Which was the leading segment in the orthotic devices pipeline market in 2023?
Orthopedic braces and supports was the leading segment in the orthotic devices pipeline market as of December 2023.
-
Which territory had the highest number of products in the orthotic devices pipeline market in 2023?
As of December 2023, the US accounted for the highest number of products in the orthotic devices pipeline market.
-
Which was the most followed regulatory pathway in the orthotic devices pipeline market in 2023?
As of December 2023, 510(k) was the most followed pathway for pipeline products in the orthotic devices market.
-
Which are the major companies operating in the orthotic devices pipeline market?
A few of the key companies associated with the orthotic devices pipeline market are AbiliTech Medical Inc, AidPlex, AIM Laboratory, Biomotum Inc, and Bionic Power Inc., among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Orthotic Devices reports