|1 Table of Contents 3
|1.1 List of Tables 20
|1.2 List of Figures 48
2 Introduction 49
2.1 Peripheral Vascular Devices Overview 49
3 Products under Development 52
3.1 Peripheral Vascular Devices – Pipeline Products by Stage of Development 52
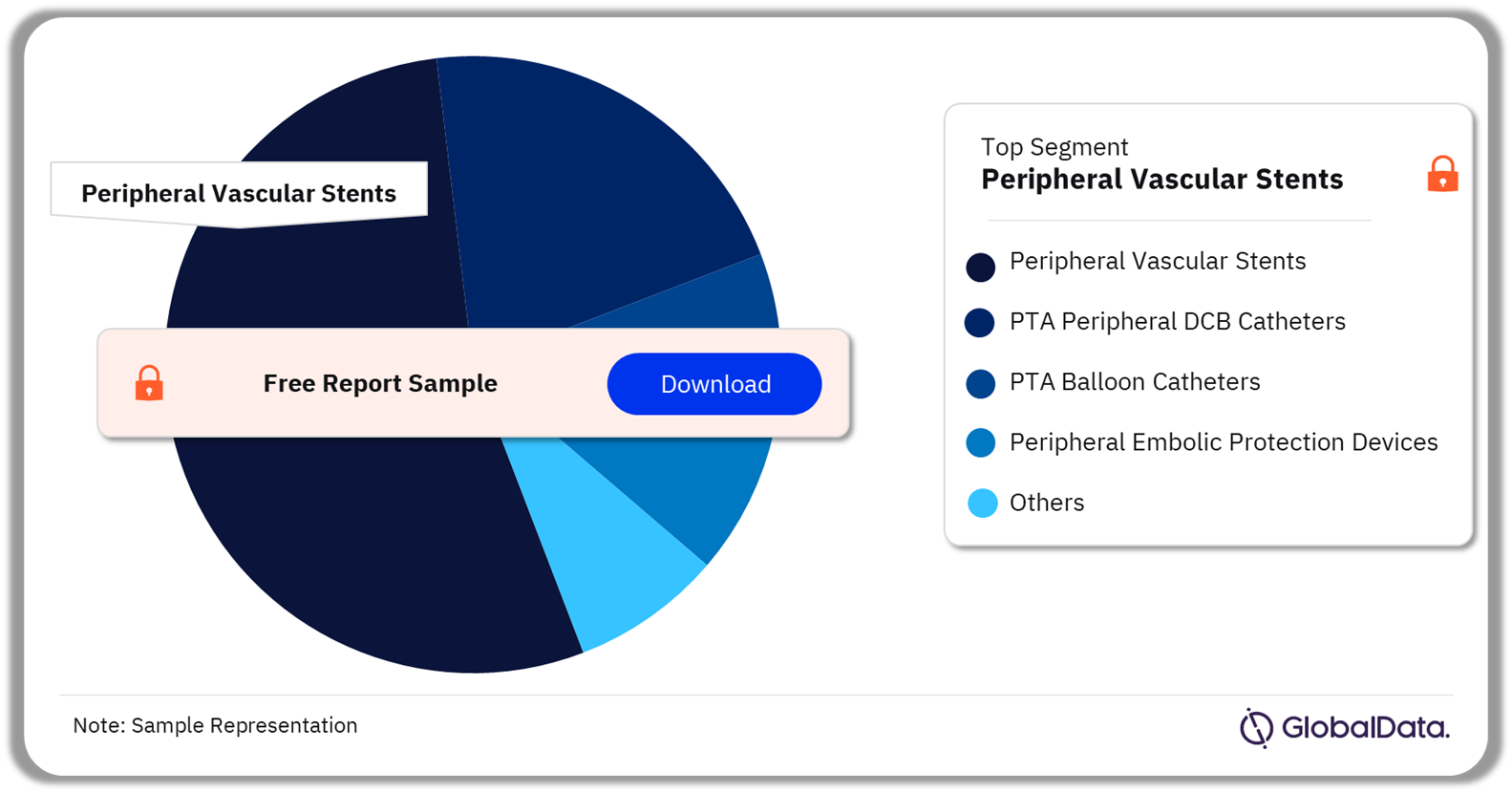
3.2 Peripheral Vascular Devices – Pipeline Products by Segment 53
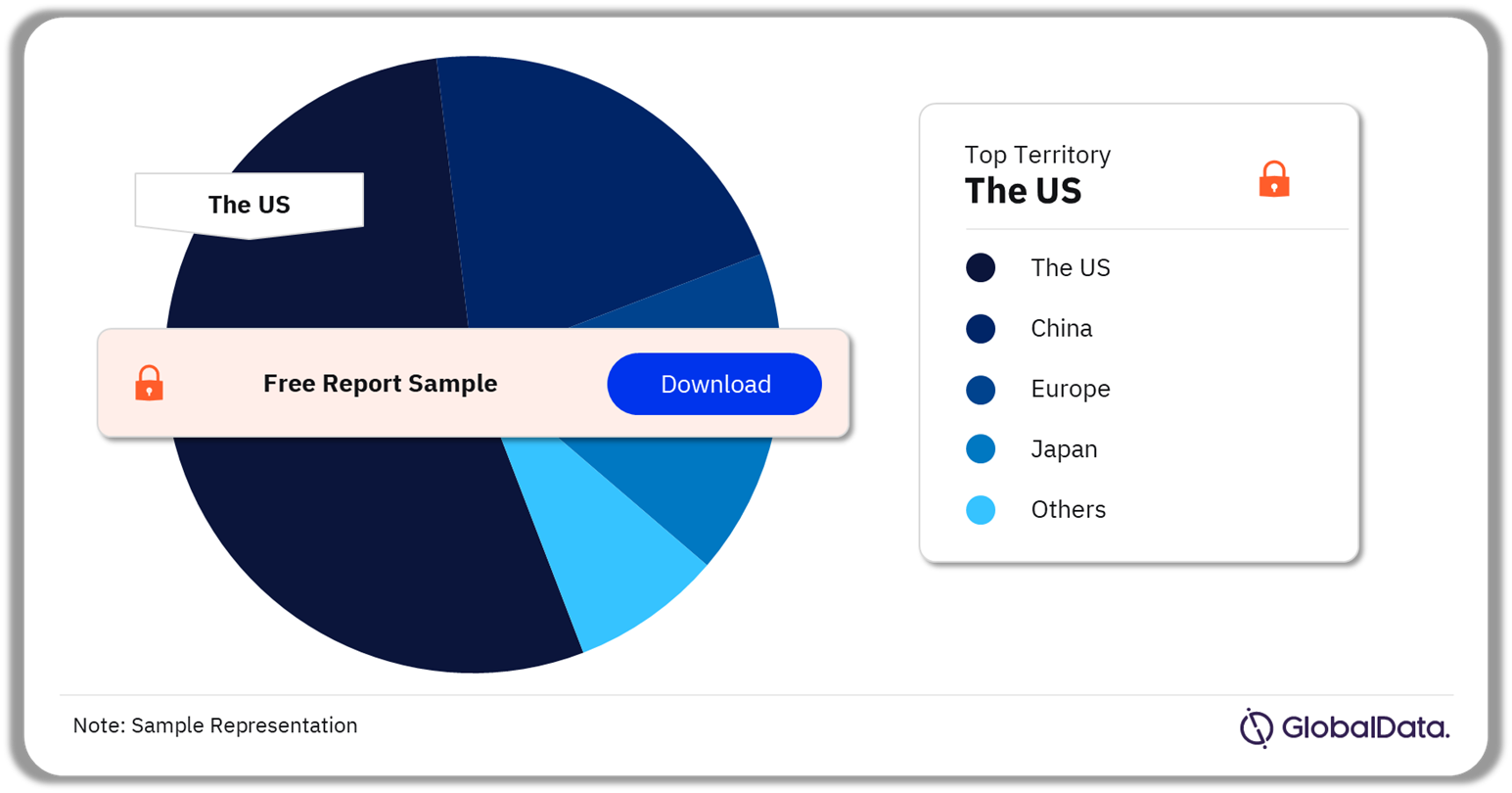
3.3 Peripheral Vascular Devices – Pipeline Products by Territory 55
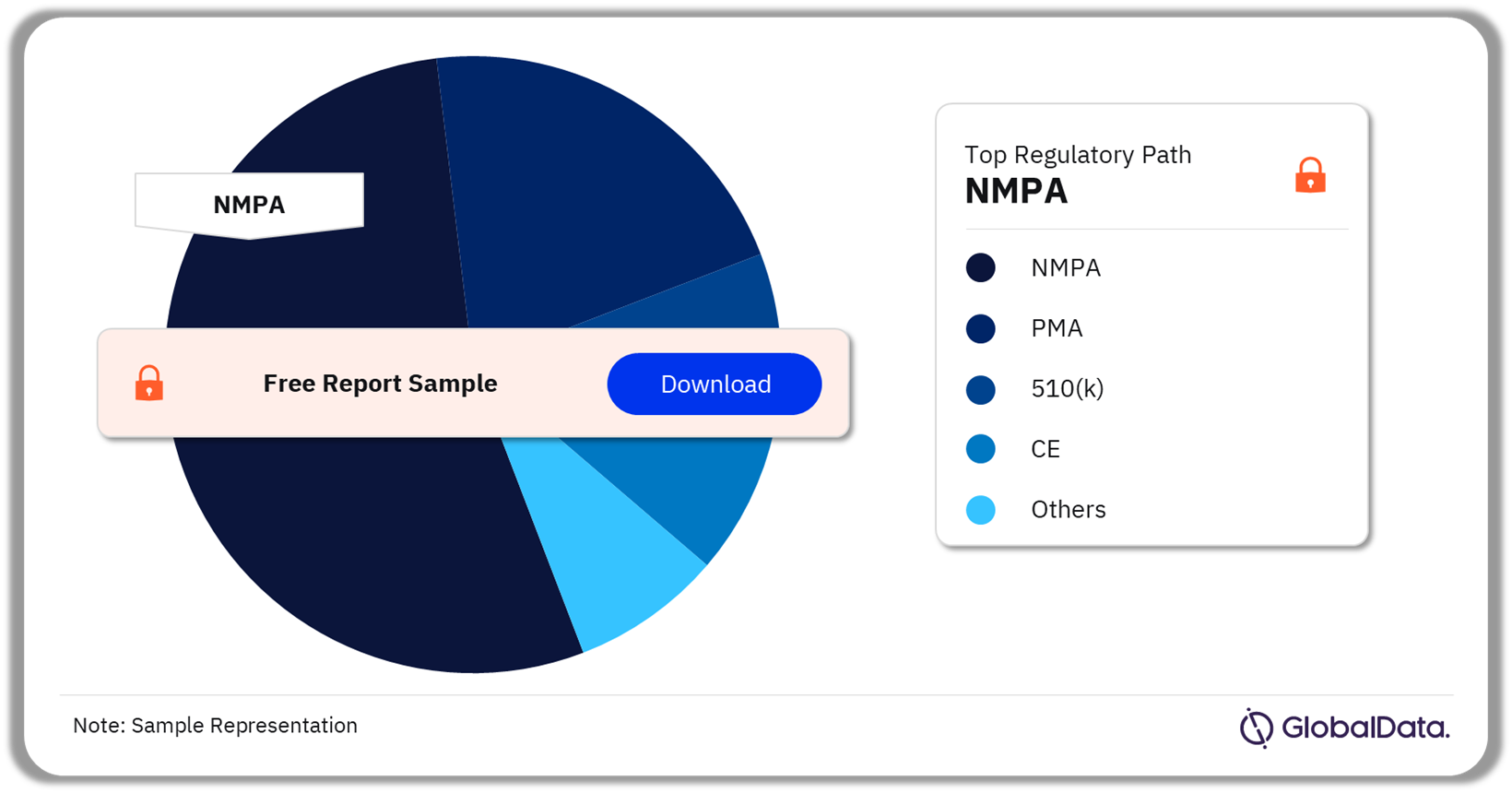
3.4 Peripheral Vascular Devices – Pipeline Products by Regulatory Path 57
3.5 Peripheral Vascular Devices – Pipeline Products by Estimated Approval Date 58
3.6 Peripheral Vascular Devices – Ongoing Clinical Trials 59
4 Peripheral Vascular Devices – Pipeline Products under Development by Companies 60
4.1 Peripheral Vascular Devices Companies – Pipeline Products by Stage of Development 60
4.2 Peripheral Vascular Devices – Pipeline Products by Stage of Development 70
5 Peripheral Vascular Devices Companies and Product Overview 81
5.1 3D Biotek LLC Company Overview 81
5.1.1 3D Biotek LLC Pipeline Products & Ongoing Clinical Trials Overview 81
5.2 Abbott Vascular Inc Company Overview 82
5.2.1 Abbott Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 82
5.3 Ablative Solutions Inc Company Overview 88
5.3.1 Ablative Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 88
5.4 Acotec Scientific Co Ltd Company Overview 91
5.4.1 Acotec Scientific Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 91
5.5 Acotec Scientific Holdings Ltd Company Overview 97
5.5.1 Acotec Scientific Holdings Ltd Pipeline Products & Ongoing Clinical Trials Overview 97
5.6 Adept Medical Ltd Company Overview 104
5.6.1 Adept Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 104
5.7 Agile Devices Inc Company Overview 105
5.7.1 Agile Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 105
5.8 Allegra Orthopaedics Ltd Company Overview 106
5.8.1 Allegra Orthopaedics Ltd Pipeline Products & Ongoing Clinical Trials Overview 106
5.9 Alucent Biomedical Inc Company Overview 107
5.9.1 Alucent Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 107
5.10 Alucent Biomedical Inc Company Overview 108
5.10.1 Alucent Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 108
5.11 amg International GmbH Company Overview 112
5.11.1 amg International GmbH Pipeline Products & Ongoing Clinical Trials Overview 112
5.12 Amplitude Vascular Systems Inc Company Overview 113
5.12.1 Amplitude Vascular Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 113
5.13 Andramed GmbH Company Overview 117
5.13.1 Andramed GmbH Pipeline Products & Ongoing Clinical Trials Overview 117
5.14 AndraTec GmbH Company Overview 119
5.14.1 AndraTec GmbH Pipeline Products & Ongoing Clinical Trials Overview 119
5.15 AnGes Inc Company Overview 120
5.15.1 AnGes Inc Pipeline Products & Ongoing Clinical Trials Overview 120
5.16 AngioCure, Inc. Company Overview 121
5.16.1 AngioCure, Inc. Pipeline Products & Ongoing Clinical Trials Overview 121
5.17 AngioDynamics Inc Company Overview 122
5.17.1 AngioDynamics Inc Pipeline Products & Ongoing Clinical Trials Overview 122
5.18 AorticLab Sarl Company Overview 124
5.18.1 AorticLab Sarl Pipeline Products & Ongoing Clinical Trials Overview 124
5.19 Aporo Biomedical, Inc. (Inactive) Company Overview 127
5.19.1 Aporo Biomedical, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 127
5.20 APT Medical Inc Company Overview 128
5.20.1 APT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 128
5.21 Arterius Ltd Company Overview 134
5.21.1 Arterius Ltd Pipeline Products & Ongoing Clinical Trials Overview 134
5.22 Artio Medical Company Overview 135
5.22.1 Artio Medical Pipeline Products & Ongoing Clinical Trials Overview 135
5.23 Artventive Medical Group Inc Company Overview 138
5.23.1 Artventive Medical Group Inc Pipeline Products & Ongoing Clinical Trials Overview 138
5.24 Ascenion GmbH Company Overview 142
5.24.1 Ascenion GmbH Pipeline Products & Ongoing Clinical Trials Overview 142
5.25 Atrium Medical Corp Company Overview 143
5.25.1 Atrium Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 143
5.26 Avalon Laboratories LLC Company Overview 144
5.26.1 Avalon Laboratories LLC Pipeline Products & Ongoing Clinical Trials Overview 144
5.27 Avantec Vascular Corp Company Overview 145
5.27.1 Avantec Vascular Corp Pipeline Products & Ongoing Clinical Trials Overview 145
5.28 Becton Dickinson and Co Company Overview 146
5.28.1 Becton Dickinson and Co Pipeline Products & Ongoing Clinical Trials Overview 146
5.29 Ben-Gurion University of the Negev Company Overview 151
5.29.1 Ben-Gurion University of the Negev Pipeline Products & Ongoing Clinical Trials Overview 151
5.30 Biotronik AG Company Overview 152
5.30.1 Biotronik AG Pipeline Products & Ongoing Clinical Trials Overview 152
5.31 Biotyx Medical (Shenzhen) Co Ltd Company Overview 153
5.31.1 Biotyx Medical (Shenzhen) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 153
5.32 Boston Scientific Corp Company Overview 156
5.32.1 Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 156
5.33 Boston Scientific Scimed Inc Company Overview 169
5.33.1 Boston Scientific Scimed Inc Pipeline Products & Ongoing Clinical Trials Overview 169
5.34 Boston TransTec LLC Company Overview 170
5.34.1 Boston TransTec LLC Pipeline Products & Ongoing Clinical Trials Overview 170
5.35 Brattea Company Overview 171
5.35.1 Brattea Pipeline Products & Ongoing Clinical Trials Overview 171
5.36 CardioDex Ltd (Inactive) Company Overview 172
5.36.1 CardioDex Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 172
5.37 Cardio-Flow Ltd. Company Overview 173
5.37.1 Cardio-Flow Ltd. Pipeline Products & Ongoing Clinical Trials Overview 173
5.38 Cardionovum GmbH Company Overview 174
5.38.1 Cardionovum GmbH Pipeline Products & Ongoing Clinical Trials Overview 174
5.39 Cardiovascular Systems Inc Company Overview 175
5.39.1 Cardiovascular Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 175
5.40 Cardiva Medical Inc Company Overview 179
5.40.1 Cardiva Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 179
5.41 Catharos Medical Systems Inc Company Overview 182
5.41.1 Catharos Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 182
5.42 CaveoMed GmbH Company Overview 183
5.42.1 CaveoMed GmbH Pipeline Products & Ongoing Clinical Trials Overview 183
5.43 Chansu Vascular Technologies LLC Company Overview 185
5.43.1 Chansu Vascular Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 185
5.44 Clemson University Company Overview 188
5.44.1 Clemson University Pipeline Products & Ongoing Clinical Trials Overview 188
5.45 CloSys Corp Company Overview 189
5.45.1 CloSys Corp Pipeline Products & Ongoing Clinical Trials Overview 189
5.46 Concept Medical Inc Company Overview 190
5.46.1 Concept Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 190
5.47 Contego Medical LLC Company Overview 195
5.47.1 Contego Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 195
5.48 Cook Medical Inc Company Overview 200
5.48.1 Cook Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 200
5.49 Cordis Corp Company Overview 204
5.49.1 Cordis Corp Pipeline Products & Ongoing Clinical Trials Overview 204
5.50 CorInnova, Inc. Company Overview 213
5.50.1 CorInnova, Inc. Pipeline Products & Ongoing Clinical Trials Overview 213
5.51 Covidien Ltd Company Overview 214
5.51.1 Covidien Ltd Pipeline Products & Ongoing Clinical Trials Overview 214
5.52 CPC of America Inc Company Overview 215
5.52.1 CPC of America Inc Pipeline Products & Ongoing Clinical Trials Overview 215
5.53 CR Bard Inc Company Overview 216
5.53.1 CR Bard Inc Pipeline Products & Ongoing Clinical Trials Overview 216
5.54 CryoMedix, LLC. Company Overview 217
5.54.1 CryoMedix, LLC. Pipeline Products & Ongoing Clinical Trials Overview 217
5.55 CyndRX LLC Company Overview 218
5.55.1 CyndRX LLC Pipeline Products & Ongoing Clinical Trials Overview 218
5.56 Cytograft Tissue Engineering Inc (Inactive) Company Overview 219
5.56.1 Cytograft Tissue Engineering Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 219
5.57 Deep Vein Medical, Inc. Company Overview 220
5.57.1 Deep Vein Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 220
5.58 Dextera Surgical Inc Company Overview 221
5.58.1 Dextera Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview 221
5.59 Dingke Medical Technology (Suzhou) Co Ltd Company Overview 222
5.59.1 Dingke Medical Technology (Suzhou) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 222
5.60 Dongguan TT Medical Inc Company Overview 223
5.60.1 Dongguan TT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 223
5.61 Dublin City University Company Overview 226
5.61.1 Dublin City University Pipeline Products & Ongoing Clinical Trials Overview 226
5.62 Echopoint Medical Ltd Company Overview 227
5.62.1 Echopoint Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 227
5.63 Edwards Lifesciences Corp Company Overview 229
5.63.1 Edwards Lifesciences Corp Pipeline Products & Ongoing Clinical Trials Overview 229
5.64 Efemoral Medical LLC Company Overview 230
5.64.1 Efemoral Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 230
5.65 Efferent Labs Inc Company Overview 234
5.65.1 Efferent Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 234
5.66 Electroformed Stents Inc Company Overview 235
5.66.1 Electroformed Stents Inc Pipeline Products & Ongoing Clinical Trials Overview 235
5.67 Elixir Medical Corp Company Overview 238
5.67.1 Elixir Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 238
5.68 Emboline, Inc. Company Overview 239
5.68.1 Emboline, Inc. Pipeline Products & Ongoing Clinical Trials Overview 239
5.69 Endocor GmbH Company Overview 242
5.69.1 Endocor GmbH Pipeline Products & Ongoing Clinical Trials Overview 242
5.70 Endomimetics LLC Company Overview 243
5.70.1 Endomimetics LLC Pipeline Products & Ongoing Clinical Trials Overview 243
5.71 EndoPhlebex Technologies, Inc Company Overview 244
5.71.1 EndoPhlebex Technologies, Inc Pipeline Products & Ongoing Clinical Trials Overview 244
5.72 enVVeno Medical Corp Company Overview 245
5.72.1 enVVeno Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 245
5.73 EpiEP, Inc. Company Overview 250
5.73.1 EpiEP, Inc. Pipeline Products & Ongoing Clinical Trials Overview 250
5.74 Eqalix, Inc. Company Overview 251
5.74.1 Eqalix, Inc. Pipeline Products & Ongoing Clinical Trials Overview 251
5.75 eucatech AG Company Overview 252
5.75.1 eucatech AG Pipeline Products & Ongoing Clinical Trials Overview 252
5.76 Ev3 Inc Company Overview 253
5.76.1 Ev3 Inc Pipeline Products & Ongoing Clinical Trials Overview 253
5.77 Evasc Medical Systems Corp Company Overview 254
5.77.1 Evasc Medical Systems Corp Pipeline Products & Ongoing Clinical Trials Overview 254
5.78 Eximo Medical Ltd Company Overview 255
5.78.1 Eximo Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 255
5.79 Filterlex Medical Ltd Company Overview 256
5.79.1 Filterlex Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 256
5.80 FIT Biotech Oy (Inactive) Company Overview 257
5.80.1 FIT Biotech Oy (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 257
5.81 Flomatrix Pty Ltd Company Overview 259
5.81.1 Flomatrix Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 259
5.82 FlowCo Inc. Company Overview 260
5.82.1 FlowCo Inc. Pipeline Products & Ongoing Clinical Trials Overview 260
5.83 Fluidx Medical Technology LLC Company Overview 261
5.83.1 Fluidx Medical Technology LLC Pipeline Products & Ongoing Clinical Trials Overview 261
5.84 Genesis MedTech International Pvt Ltd Company Overview 262
5.84.1 Genesis MedTech International Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 262
5.85 George Washington University Company Overview 265
5.85.1 George Washington University Pipeline Products & Ongoing Clinical Trials Overview 265
5.86 Gradient Denervation Technologies Company Overview 266
5.86.1 Gradient Denervation Technologies Pipeline Products & Ongoing Clinical Trials Overview 266
5.87 Guerbet SA Company Overview 270
5.87.1 Guerbet SA Pipeline Products & Ongoing Clinical Trials Overview 270
5.88 Hadassah Medical Center Company Overview 273
5.88.1 Hadassah Medical Center Pipeline Products & Ongoing Clinical Trials Overview 273
5.89 Handok-Kalos Medical Company Overview 274
5.89.1 Handok-Kalos Medical Pipeline Products & Ongoing Clinical Trials Overview 274
5.90 Hansen Medical Inc (Inactive) Company Overview 277
5.90.1 Hansen Medical Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 277
5.91 Harvard University Company Overview 278
5.91.1 Harvard University Pipeline Products & Ongoing Clinical Trials Overview 278
5.92 HeMo Bioengineering Ltd Company Overview 279
5.92.1 HeMo Bioengineering Ltd Pipeline Products & Ongoing Clinical Trials Overview 279
5.93 Hexacath SA Company Overview 283
5.93.1 Hexacath SA Pipeline Products & Ongoing Clinical Trials Overview 283
5.94 ID Nest Medical SAS Company Overview 284
5.94.1 ID Nest Medical SAS Pipeline Products & Ongoing Clinical Trials Overview 284
5.95 Inari Medical Inc Company Overview 285
5.95.1 Inari Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 285
5.96 InnoRa GmbH Company Overview 286
5.96.1 InnoRa GmbH Pipeline Products & Ongoing Clinical Trials Overview 286
5.97 Innova Vascular Inc Company Overview 287
5.97.1 Innova Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 287
5.98 Innovative Cardiovascular Solutions, LLC Company Overview 289
5.98.1 Innovative Cardiovascular Solutions, LLC Pipeline Products & Ongoing Clinical Trials Overview 289
5.99 Innovein Medical Inc Company Overview 292
5.99.1 Innovein Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 292
5.100 Innovia LLC Company Overview 295
5.100.1 Innovia LLC Pipeline Products & Ongoing Clinical Trials Overview 295
5.101 InspireMD Inc Company Overview 297
5.101.1 InspireMD Inc Pipeline Products & Ongoing Clinical Trials Overview 297
5.102 Instylla Inc Company Overview 304
5.102.1 Instylla Inc Pipeline Products & Ongoing Clinical Trials Overview 304
5.103 International Cardio Corp Company Overview 307
5.103.1 International Cardio Corp Pipeline Products & Ongoing Clinical Trials Overview 307
5.104 InterVene Inc. Company Overview 310
5.104.1 InterVene Inc. Pipeline Products & Ongoing Clinical Trials Overview 310
5.105 Intressa Vascular SA Company Overview 313
5.105.1 Intressa Vascular SA Pipeline Products & Ongoing Clinical Trials Overview 313
5.106 InVera Medical Ltd Company Overview 314
5.106.1 InVera Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 314
5.107 IPI Singapore Company Overview 315
5.107.1 IPI Singapore Pipeline Products & Ongoing Clinical Trials Overview 315
5.108 Javelin Medical Ltd Company Overview 316
5.108.1 Javelin Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 316
5.109 Johns Hopkins University Company Overview 319
5.109.1 Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 319
5.110 Keystone Heart Ltd Company Overview 320
5.110.1 Keystone Heart Ltd Pipeline Products & Ongoing Clinical Trials Overview 320
5.111 Kona Medical Inc Company Overview 321
5.111.1 Kona Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 321
5.112 Kyoto Medical Planning Co Ltd Company Overview 322
5.112.1 Kyoto Medical Planning Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 322
5.113 LeMaitre Vascular Inc Company Overview 324
5.113.1 LeMaitre Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 324
5.114 LeoMed, LLC Company Overview 326
5.114.1 LeoMed, LLC Pipeline Products & Ongoing Clinical Trials Overview 326
5.115 Lepu Medical Technology (Beijing) Co Ltd Company Overview 327
5.115.1 Lepu Medical Technology (Beijing) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 327
5.116 Lepu Scientech Medical Technology (Shanghai) Co Ltd Company Overview 330
5.116.1 Lepu Scientech Medical Technology (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 330
5.117 Lifetech Scientific (Shenzhen) Co Ltd Company Overview 331
5.117.1 Lifetech Scientific (Shenzhen) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 331
5.118 LimFlow SA Company Overview 335
5.118.1 LimFlow SA Pipeline Products & Ongoing Clinical Trials Overview 335
5.119 LuSeed Vascular Company Overview 339
5.119.1 LuSeed Vascular Pipeline Products & Ongoing Clinical Trials Overview 339
5.120 Lutonix Inc Company Overview 340
5.120.1 Lutonix Inc Pipeline Products & Ongoing Clinical Trials Overview 340
5.121 Lyra Therapeutics Inc Company Overview 341
5.121.1 Lyra Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 341
5.122 Marblehead Medical LLC Company Overview 343
5.122.1 Marblehead Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 343
5.123 Mayo Clinic Company Overview 344
5.123.1 Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 344
5.124 Medical Device Creations Ltd. Company Overview 345
5.124.1 Medical Device Creations Ltd. Pipeline Products & Ongoing Clinical Trials Overview 345
5.125 Medikit Co Ltd Company Overview 346
5.125.1 Medikit Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 346
5.126 Medtronic Plc Company Overview 347
5.126.1 Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 347
5.127 Mercator MedSystems Inc Company Overview 355
5.127.1 Mercator MedSystems Inc Pipeline Products & Ongoing Clinical Trials Overview 355
5.128 Meril Life Sciences Pvt Ltd Company Overview 358
5.128.1 Meril Life Sciences Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 358
5.129 Micell Technologies Inc Company Overview 363
5.129.1 Micell Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 363
5.130 Micro Medical Solutions Inc Company Overview 365
5.130.1 Micro Medical Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 365
5.131 MicroPort CardioFlow Medtech Corp Company Overview 368
5.131.1 MicroPort CardioFlow Medtech Corp Pipeline Products & Ongoing Clinical Trials Overview 368
5.132 MicroPort Scientific Corp Company Overview 369
5.132.1 MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 369
5.133 MIV Therapeutics Inc Company Overview 374
5.133.1 MIV Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 374
5.134 Nanjing SealMed Medical Technology Corp Ltd Company Overview 375
5.134.1 Nanjing SealMed Medical Technology Corp Ltd Pipeline Products & Ongoing Clinical Trials Overview 375
5.135 Nanjing Xinke Medical Devices Co Ltd Company Overview 376
5.135.1 Nanjing Xinke Medical Devices Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 376
5.136 Natec Medical Ltd Company Overview 377
5.136.1 Natec Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 377
5.137 Navi Medical Technologies Pty Ltd Company Overview 378
5.137.1 Navi Medical Technologies Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 378
5.138 Navis Medical Company Overview 381
5.138.1 Navis Medical Pipeline Products & Ongoing Clinical Trials Overview 381
5.139 Neovasc Inc Company Overview 382
5.139.1 Neovasc Inc Pipeline Products & Ongoing Clinical Trials Overview 382
5.140 Nesstent Ltd. Company Overview 383
5.140.1 Nesstent Ltd. Pipeline Products & Ongoing Clinical Trials Overview 383
5.141 Neurosonix Ltd. Company Overview 384
5.141.1 Neurosonix Ltd. Pipeline Products & Ongoing Clinical Trials Overview 384
5.142 NewMed Medical Co Ltd Company Overview 385
5.142.1 NewMed Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 385
5.143 NIH Office of Technology Transfer Company Overview 387
5.143.1 NIH Office of Technology Transfer Pipeline Products & Ongoing Clinical Trials Overview 387
5.144 Nipro Corp Company Overview 388
5.144.1 Nipro Corp Pipeline Products & Ongoing Clinical Trials Overview 388
5.145 Northwestern University Company Overview 389
5.145.1 Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 389
5.146 Northwind Medical Inc Company Overview 390
5.146.1 Northwind Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 390
5.147 NovaPulse Ltd Company Overview 391
5.147.1 NovaPulse Ltd Pipeline Products & Ongoing Clinical Trials Overview 391
5.148 Novoheart Holdings Inc Company Overview 392
5.148.1 Novoheart Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 392
5.149 NSVascular, Inc. Company Overview 393
5.149.1 NSVascular, Inc. Pipeline Products & Ongoing Clinical Trials Overview 393
5.150 NuVascular Technologies Inc Company Overview 394
5.150.1 NuVascular Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 394
5.151 OrbusNeich Company Overview 395
5.151.1 OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview 395
5.152 Orchestra BioMed Inc Company Overview 400
5.152.1 Orchestra BioMed Inc Pipeline Products & Ongoing Clinical Trials Overview 400
5.153 Oregon Health & Science University Company Overview 401
5.153.1 Oregon Health & Science University Pipeline Products & Ongoing Clinical Trials Overview 401
5.154 Palmaz Scientific Inc (Inactive) Company Overview 403
5.154.1 Palmaz Scientific Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 403
5.155 PerAssist Ltd Company Overview 404
5.155.1 PerAssist Ltd Pipeline Products & Ongoing Clinical Trials Overview 404
5.156 PeriTec Biosciences Ltd Company Overview 405
5.156.1 PeriTec Biosciences Ltd Pipeline Products & Ongoing Clinical Trials Overview 405
5.157 Perseus Biomed Ltd Company Overview 407
5.157.1 Perseus Biomed Ltd Pipeline Products & Ongoing Clinical Trials Overview 407
5.158 PQ Bypass Inc Company Overview 408
5.158.1 PQ Bypass Inc Pipeline Products & Ongoing Clinical Trials Overview 408
5.159 Praxis Medical Devices Ltd Company Overview 410
5.159.1 Praxis Medical Devices Ltd Pipeline Products & Ongoing Clinical Trials Overview 410
5.160 ProMed Inc Company Overview 413
5.160.1 ProMed Inc Pipeline Products & Ongoing Clinical Trials Overview 413
5.161 Protembis GmbH Company Overview 414
5.161.1 Protembis GmbH Pipeline Products & Ongoing Clinical Trials Overview 414
5.162 Pulsus Medical LLC (Inactive) Company Overview 417
5.162.1 Pulsus Medical LLC (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 417
5.163 QT Vascular Ltd Company Overview 418
5.163.1 QT Vascular Ltd Pipeline Products & Ongoing Clinical Trials Overview 418
5.164 QualiMed Innovative Medizinprodukte GmbH Company Overview 419
5.164.1 QualiMed Innovative Medizinprodukte GmbH Pipeline Products & Ongoing Clinical Trials Overview 419
5.165 Qvanteq AG Company Overview 422
5.165.1 Qvanteq AG Pipeline Products & Ongoing Clinical Trials Overview 422
5.166 R3 Vascular Inc Company Overview 423
5.166.1 R3 Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 423
5.167 RadSense Company Overview 426
5.167.1 RadSense Pipeline Products & Ongoing Clinical Trials Overview 426
5.168 Reflow Medical, Inc. Company Overview 427
5.168.1 Reflow Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 427
5.169 RegenMedTX LLC Company Overview 431
5.169.1 RegenMedTX LLC Pipeline Products & Ongoing Clinical Trials Overview 431
5.170 Renal Dynamics, LLC Company Overview 432
5.170.1 Renal Dynamics, LLC Pipeline Products & Ongoing Clinical Trials Overview 432
5.171 Renaly Ltd Company Overview 433
5.171.1 Renaly Ltd Pipeline Products & Ongoing Clinical Trials Overview 433
5.172 Reprieve Cardiovascular Inc Company Overview 434
5.172.1 Reprieve Cardiovascular Inc Pipeline Products & Ongoing Clinical Trials Overview 434
5.173 REVA Medical Inc Company Overview 437
5.173.1 REVA Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 437
5.174 Rex Medical LP Company Overview 440
5.174.1 Rex Medical LP Pipeline Products & Ongoing Clinical Trials Overview 440
5.175 Rontis AG Company Overview 442
5.175.1 Rontis AG Pipeline Products & Ongoing Clinical Trials Overview 442
5.176 Rutgers The State University of New Jersey Company Overview 443
5.176.1 Rutgers The State University of New Jersey Pipeline Products & Ongoing Clinical Trials Overview 443
5.177 Shanghai AngioCare Medical Technology Co Ltd Company Overview 444
5.177.1 Shanghai AngioCare Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 444
5.178 Shanghai Bio-heart Biological Technology Co Ltd Company Overview 446
5.178.1 Shanghai Bio-heart Biological Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 446
5.179 Shanghai Changde Medical Technology Co Ltd Company Overview 448
5.179.1 Shanghai Changde Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 448
5.180 Shanghai Golden Leaf Medtech Co Ltd Company Overview 456
5.180.1 Shanghai Golden Leaf Medtech Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 456
5.181 Shanghai Healing Medical Instruments Co Ltd Company Overview 457
5.181.1 Shanghai Healing Medical Instruments Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 457
5.182 Shanghai HeartCare Medical Technology Co Ltd Company Overview 458
5.182.1 Shanghai HeartCare Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 458
5.183 Shanghai MicroPort Endovascular MedTech Group Co Ltd Company Overview 459
5.183.1 Shanghai MicroPort Endovascular MedTech Group Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 459
5.184 Shanghai Shenqi Medical Technology Co Ltd Company Overview 464
5.184.1 Shanghai Shenqi Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 464
5.185 Shanghai Xuanyu Medical Equipment Co Ltd Company Overview 465
5.185.1 Shanghai Xuanyu Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 465
5.186 Shape Memory Medical Inc Company Overview 466
5.186.1 Shape Memory Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 466
5.187 Shenzhen Salubris Pharmaceuticals Co Ltd Company Overview 469
5.187.1 Shenzhen Salubris Pharmaceuticals Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 469
5.188 Shockwave Medical Inc Company Overview 477
5.188.1 Shockwave Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 477
5.189 SiL Vascular Ltd Company Overview 480
5.189.1 SiL Vascular Ltd Pipeline Products & Ongoing Clinical Trials Overview 480
5.190 SoniVie Ltd. Company Overview 481
5.190.1 SoniVie Ltd. Pipeline Products & Ongoing Clinical Trials Overview 481
5.191 SonoVascular Inc Company Overview 484
5.191.1 SonoVascular Inc Pipeline Products & Ongoing Clinical Trials Overview 484
5.192 Sound Interventions, Inc. Company Overview 485
5.192.1 Sound Interventions, Inc. Pipeline Products & Ongoing Clinical Trials Overview 485
5.193 SoundBite Medical Solutions Inc Company Overview 486
5.193.1 SoundBite Medical Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 486
5.194 Spectranetics Corp Company Overview 490
5.194.1 Spectranetics Corp Pipeline Products & Ongoing Clinical Trials Overview 490
5.195 SpectroCon LLC Company Overview 491
5.195.1 SpectroCon LLC Pipeline Products & Ongoing Clinical Trials Overview 491
5.196 Spectrumedics Medical Technology (Shanghai) Co Ltd Company Overview 492
5.196.1 Spectrumedics Medical Technology (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 492
5.197 Sree Chitra Tirunal Institute for Medical Sciences & Technology Company Overview 493
5.197.1 Sree Chitra Tirunal Institute for Medical Sciences & Technology Pipeline Products & Ongoing Clinical Trials Overview 493
5.198 St. Jude Medical LLC Company Overview 494
5.198.1 St. Jude Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 494
5.199 Stanford University Company Overview 495
5.199.1 Stanford University Pipeline Products & Ongoing Clinical Trials Overview 495
5.200 Stemplant LLC Company Overview 498
5.200.1 Stemplant LLC Pipeline Products & Ongoing Clinical Trials Overview 498
5.201 Straub Medical AG Company Overview 499
5.201.1 Straub Medical AG Pipeline Products & Ongoing Clinical Trials Overview 499
5.202 SurModics Inc Company Overview 502
5.202.1 SurModics Inc Pipeline Products & Ongoing Clinical Trials Overview 502
5.203 Suzhou Rainmed Medical Technology Co Ltd Company Overview 510
5.203.1 Suzhou Rainmed Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 510
5.204 Suzhou Xinmai Medical Equipment Co Ltd Company Overview 511
5.204.1 Suzhou Xinmai Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 511
5.205 Suzhou Yinluo Medical Devices Co Ltd Company Overview 515
5.205.1 Suzhou Yinluo Medical Devices Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 515
5.206 Suzhou Zhonghui Medical Technology Co Ltd Company Overview 516
5.206.1 Suzhou Zhonghui Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 516
5.207 Symple Surgical Inc Company Overview 519
5.207.1 Symple Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview 519
5.208 Synergy Flow Ltd Company Overview 520
5.208.1 Synergy Flow Ltd Pipeline Products & Ongoing Clinical Trials Overview 520
5.209 Tailored Medical Devices Inc Company Overview 521
5.209.1 Tailored Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 521
5.210 Tel Aviv University Company Overview 522
5.210.1 Tel Aviv University Pipeline Products & Ongoing Clinical Trials Overview 522
5.211 Teleflex Inc Company Overview 523
5.211.1 Teleflex Inc Pipeline Products & Ongoing Clinical Trials Overview 523
5.212 Tepha Inc Company Overview 525
5.212.1 Tepha Inc Pipeline Products & Ongoing Clinical Trials Overview 525
5.213 Terumo Corp Company Overview 526
5.213.1 Terumo Corp Pipeline Products & Ongoing Clinical Trials Overview 526
5.214 Terumo Interventional Systems Company Overview 529
5.214.1 Terumo Interventional Systems Pipeline Products & Ongoing Clinical Trials Overview 529
5.215 Thrombx Medical Inc Company Overview 531
5.215.1 Thrombx Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 531
5.216 TissueGen Inc Company Overview 534
5.216.1 TissueGen Inc Pipeline Products & Ongoing Clinical Trials Overview 534
5.217 Tractivus SL Company Overview 535
5.217.1 Tractivus SL Pipeline Products & Ongoing Clinical Trials Overview 535
5.218 Transit Scientific LLC Company Overview 536
5.218.1 Transit Scientific LLC Pipeline Products & Ongoing Clinical Trials Overview 536
5.219 TransMed7 LLC Company Overview 539
5.219.1 TransMed7 LLC Pipeline Products & Ongoing Clinical Trials Overview 539
5.220 Trinity College Dublin Company Overview 540
5.220.1 Trinity College Dublin Pipeline Products & Ongoing Clinical Trials Overview 540
5.221 TripleMed BV Company Overview 541
5.221.1 TripleMed BV Pipeline Products & Ongoing Clinical Trials Overview 541
5.222 Ts3 Medical, Inc. Company Overview 544
5.222.1 Ts3 Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 544
5.223 University of Colorado Company Overview 546
5.223.1 University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 546
5.224 University of Illinois at Chicago Company Overview 547
5.224.1 University of Illinois at Chicago Pipeline Products & Ongoing Clinical Trials Overview 547
5.225 University of Louisville Company Overview 548
5.225.1 University of Louisville Pipeline Products & Ongoing Clinical Trials Overview 548
5.226 University of Michigan Company Overview 549
5.226.1 University of Michigan Pipeline Products & Ongoing Clinical Trials Overview 549
5.227 University of Minnesota Company Overview 550
5.227.1 University of Minnesota Pipeline Products & Ongoing Clinical Trials Overview 550
5.228 University of Montana Company Overview 553
5.228.1 University of Montana Pipeline Products & Ongoing Clinical Trials Overview 553
5.229 University of Nebraska Company Overview 554
5.229.1 University of Nebraska Pipeline Products & Ongoing Clinical Trials Overview 554
5.230 University of New Mexico Company Overview 555
5.230.1 University of New Mexico Pipeline Products & Ongoing Clinical Trials Overview 555
5.231 University of Pittsburgh Company Overview 556
5.231.1 University of Pittsburgh Pipeline Products & Ongoing Clinical Trials Overview 556
5.232 University of Pittsburgh Medical Center Company Overview 559
5.232.1 University of Pittsburgh Medical Center Pipeline Products & Ongoing Clinical Trials Overview 559
5.233 University of Rochester Company Overview 560
5.233.1 University of Rochester Pipeline Products & Ongoing Clinical Trials Overview 560
5.234 University of Sydney Company Overvi
![]()