Point of Care (POC) Analyzers Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Point of Care (POC) Analyzers Pipeline Market Report Overview
POC analyzers are small handheld or bench-top analyzers often used in small laboratories and physician labs with less workload and daily sample inflow. They often use disposable test cartridges and can provide faster results. The Point of Care (POC) analyzers pipeline market research report provides a comprehensive understanding of the pipeline products along with their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Segments | · POC Hand Held Hematology Analyzers
· POC Immunochemistry Analyzers · POC PCR Systems |
| Key Territories | · The US
· Europe · Canada · China · India |
| Key Regulatory Paths | · 510(k)
· CE-IVD · MDL · NMPA · ICAC |
| Leading Companies | · Access Bio Inc
· Acousort AB · Acrongenomics Inc (Inactive) · AgPlus Diagnostics LTD · Akonni Biosystems Inc |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Point of Care (POC) Analyzers Pipeline Market by Segments
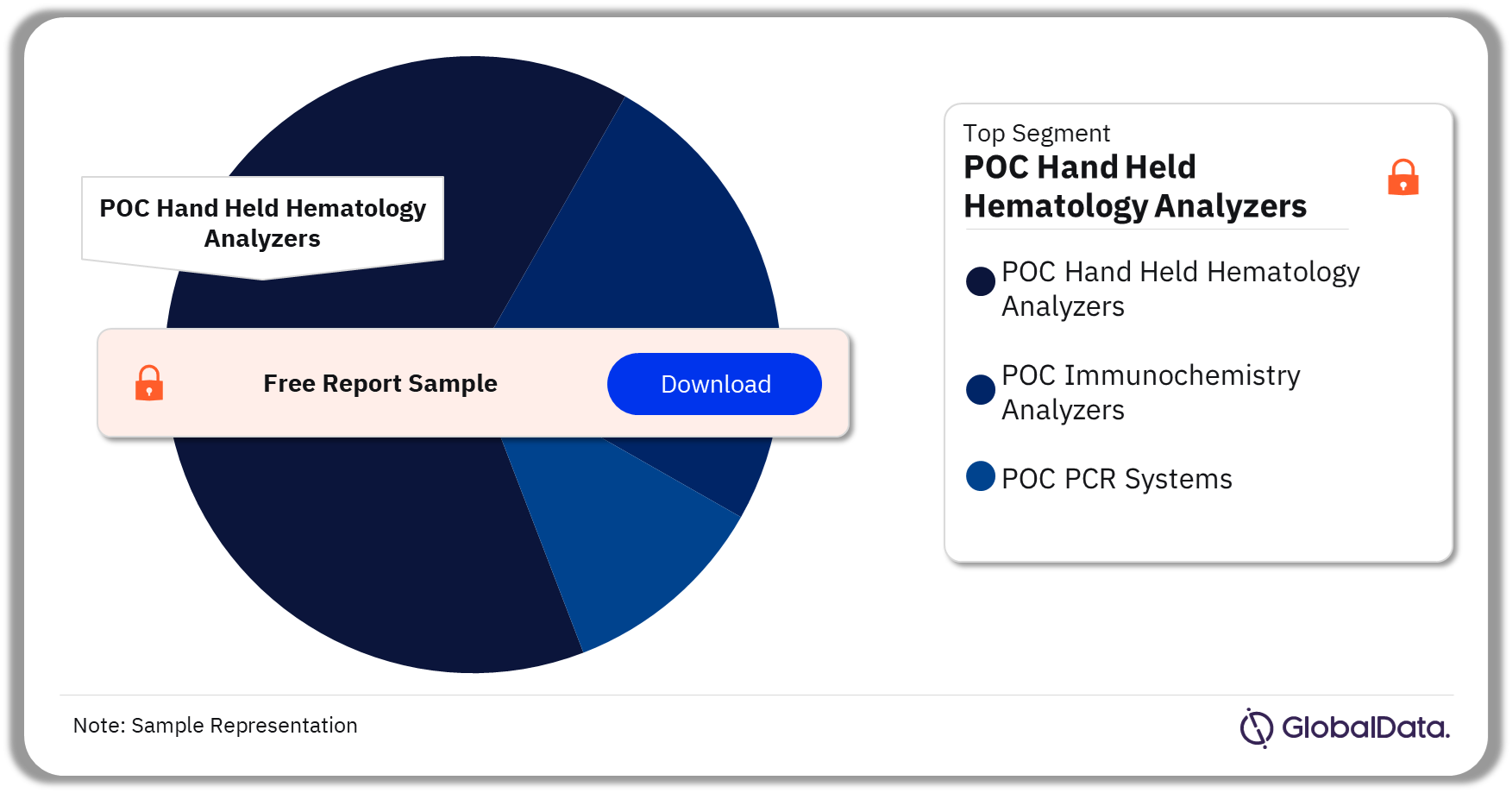
The key segments in the Point of Care (POC) analyzers pipeline market are POC Hand Held Hematology Analyzers, POC Immunochemistry Analyzers, and POC PCR Systems. As of December 2023, POC Hand Held Hematology Analyzers accounted for the highest number of pipeline products. POC Hematology Analyzers are small hand-held analyzers used to perform tests related to blood and blood components. These analyzers are also used to identify and determine the morphology of blood and blood-forming cells.
Point of Care (POC) Analyzers Pipeline Market Analysis by Segments, 2023 (%)
Buy the Full Report for More Segment Insights into the Point of Care (POC) Analyzers Pipeline Market
Point of Care (POC) Analyzers Pipeline Market Segmentation by Territories
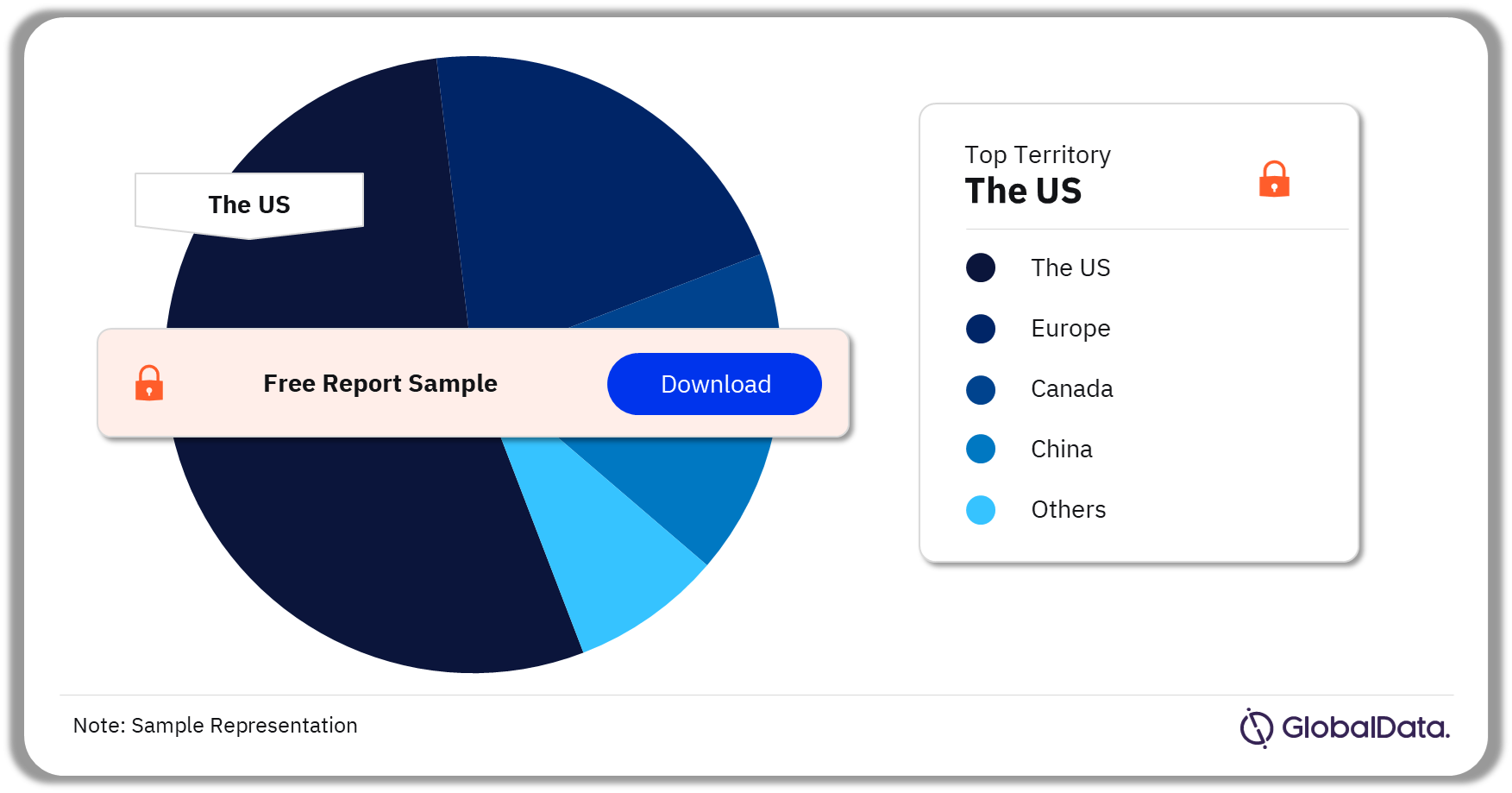
A few of the key territories for Point of Care (POC) analyzers pipeline products are the US, Europe, Canada, China, and India. As of December 2023, the US accounted for the highest number of pipeline products.
Point of Care (POC) Analyzers Pipeline Market Analysis by Territories, 2023 (%)
Buy the Full Report for More Territory Insights into the Point of Care (POC) Analyzers Pipeline Market
Point of Care (POC) Analyzers Pipeline Market Segmentation by Regulatory Paths
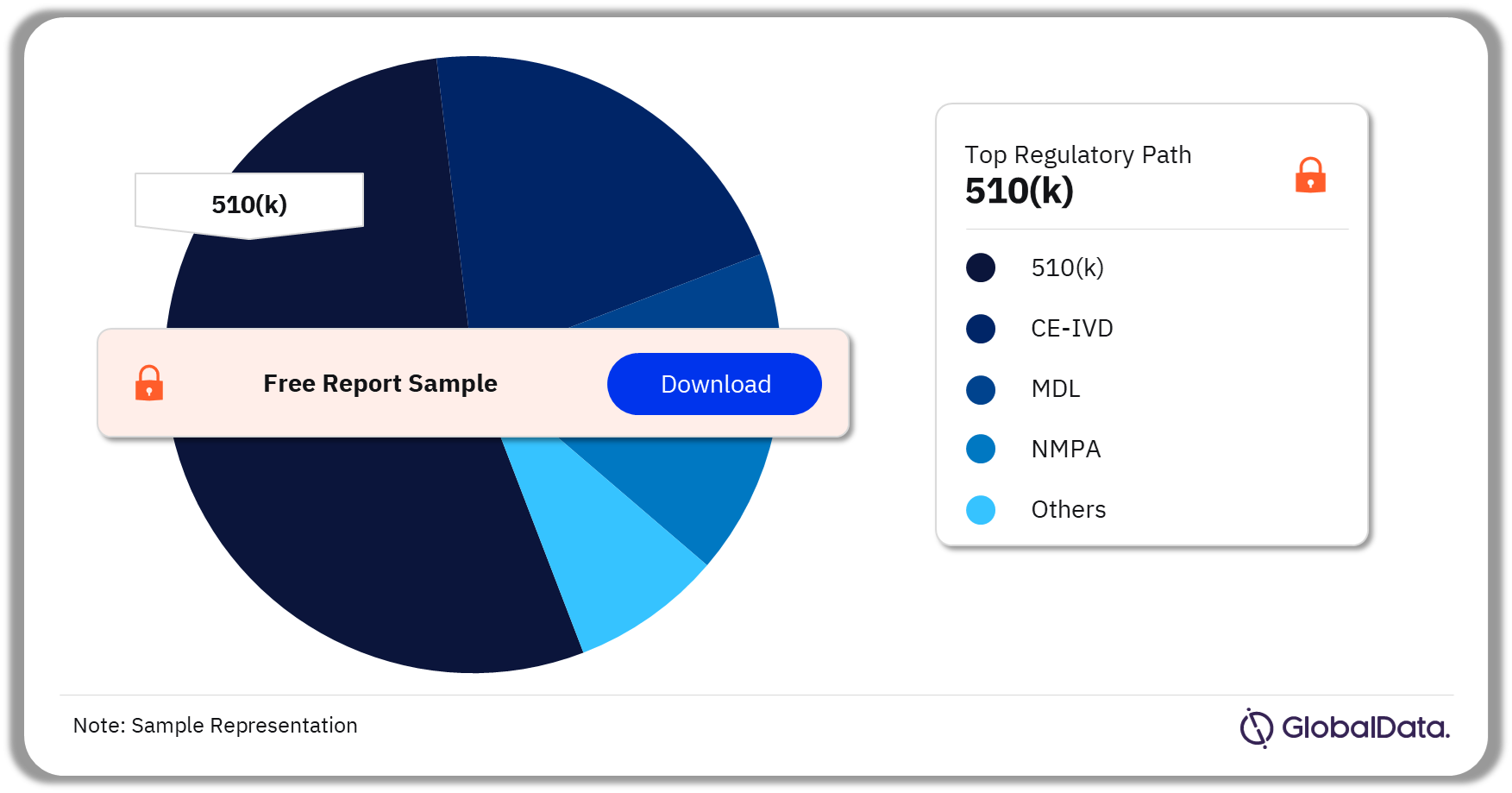
A few of the key regulatory paths followed by the Point of Care (POC) analyzers pipeline market are 510(k), CE-IVD, MDL, NMPA, and ICAC. As of December 2023, 510(k) was the most followed pathway for pipeline products in the market.
Point of Care (POC) Analyzers Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy the Full Report for More Regulatory Path Insights into the Point of Care (POC) Analyzers Pipeline Market
Point of Care (POC) Analyzers Pipeline Market – Competitive Landscape
A few of the key companies associated with the Point of Care (POC) analyzers pipeline market are Access Bio Inc., Acousort AB, Acrongenomics Inc. (Inactive), AgPlus Diagnostics LTD, and Akonni Biosystems Inc., among others.
Access Bio Inc: Access Bio is headquartered in Somerset, New Jersey, the US. It operates through its research and development and production facility located in Seoul, Korea. Besides the US and Korea, the company has a presence in Ethiopia as well. Access Bio is a medical device company that researches, develops, manufactures, and markets in-vitro diagnostics tests, biosensors, and molecular diagnostic products. The company serves non-profit organizations and private companies.
Akonni Biosystems Inc: Akonni Biosystems is headquartered in Frederick, Maryland, the US. It is a molecular diagnostics company that develops, manufactures, and distributes integrated molecular diagnostic systems. The company products find applications in therapeutical areas of infectious disease, oncology, and pharmacogenomics, among others. Akonni Biosystems partners with laboratories, biodefense companies, and universities.
Point of Care (POC) Analyzers Pipeline Market Analysis by Companies, 2023
Buy the Full Report for More Company Insights into the Point of Care (POC) Analyzers Pipeline Market
Segments Covered in the Report
Point of Care (POC) Analyzers Pipeline Market Segments Outlook
- POC Hand Held Hematology Analyzers
- POC Immunochemistry Analyzers
- POC PCR Systems
Point of Care (POC) Analyzers Pipeline Market Territories Outlook
- The US
- Europe
- Canada
- China
- India
Point of Care (POC) Analyzers Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE-IVD
- MDL
- NMPA
- ICAC
Scope
This report provides:
- Extensive coverage of the Point of Care (POC) Analyzers under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of Point of Care (POC) analyzers and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report will enable you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Point of Care (POC) analyzers under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Acousort AB
Acrongenomics Inc (Inactive)
AgPlus Diagnostics LTD
Akonni Biosystems Inc
Alere Inc
All Medicus Co Ltd
Analytical Diagnostic Solutions Inc
ApoCell Inc
Aptatek BioSciences Inc
AquaBioChip, LLC
Atonomics A/S (Inactive)
Aviv Biomedical, Inc.
Besst Test
Binghamton University
Bio-AMD Inc
BioMedomics Inc
Biopico Systems Inc
Biosensia Ltd
Boston University
BreviTest Technologies LLC
Brigham and Women's Hospital
Canon USA Inc
Center for Innovation in Point-of-Care Technologies for HIV/AIDS
ChipCare Corp
Chrogene Aarogyam Biotech Pvt Ltd
Chronus Health Inc
City, University of London
Co-Diagnostics Inc
Cornell University
Curetis GmbH
Duke University
Edan Instruments Inc
EKF Diagnostics Holdings Plc
Electrocyt, Inc.
Emergent Detection Inc (Inactive)
Endeavor Sciences Inc
Entegrion Inc
ExcitePCR Corp
Fable Biyoteknoloji San ve Tic AS
Fawkes Biotechnology LLC
First Light Diagnostics Inc
Florida International University
Future Diagnostics Solutions BV
General Fluidics Corp
Giner Inc
GlucoSentient Inc
Glyconics Ltd
Haemokinesis Pty Ltd
HemoPalm Corp
IdbyDNA Inc
Indian Institute of Science
Intelligent Optical Systems, Inc.
Invivomon Inc (Inactive)
I-Sens Inc
LabNow Inc (Inactive)
LightDeck Diagnostics Inc
Louisiana State University
Lucendi Inc
Masaryk University
Maxygen-mobile DNA tests
McKesson Corp
Memorial Sloan Kettering Cancer Center
MobioSense Inc
MyMD Pharmaceuticals Inc
Nalia Systems Ltd
Nanomix Inc
NeuroCatch Inc
Newcastle University
Nikkiso Co Ltd
Nova Biomedical Corp
Novel Microdevices LLC
Ohmx Corp
OncoGenesis
Ontera Inc (Inactive)
Oregon State University
Pacific Nanoscience, Inc.
POC Medical Systems Inc
Prominex Inc
Qvella Corp
Radisens Diagnostics Ltd
Rapid Diagnostic Pvt Ltd
RMIT University
Samvardhana Motherson Health Solution Ltd
Scryb Inc
SeLux Diagnostics Inc
SenGenix Inc
SepTec
Smiths Detection Inc
StemTek Therapeutics SL
Sugentech Inc
Thermal Gradient Inc
ThreeFold Sensors (Inactive)
Trinity Biotech Plc
University of Calgary
University of California Berkeley
University of Glasgow
University of Illinois at Urbana-Champaign
University of Pittsburgh
Ural Federal University
ViroGates AS
Wellstat Diagnostics LLC
Table of Contents
Table
Figures
Frequently asked questions
-
Which was the leading segment in the Point of Care (POC) analyzers pipeline market?
POC Hand Held Hematology Analyzers was the leading segment in the Point of Care (POC) analyzers pipeline market as of December 2023.
-
Which territory had the highest number of products in the Point of Care (POC) analyzers pipeline market?
As of December 2023, the US accounted for the highest number of products in the Point of Care (POC) analyzers pipeline market.
-
Which was the most followed regulatory pathway in the Point of Care (POC) analyzers pipeline market?
As of December 2023, 510(k) was the most followed pathway for pipeline products in the Point of Care (POC) analyzers market.
-
Which are the major companies operating in the Point of Care (POC) analyzers pipeline market?
A few of the key companies associated with the Point of Care (POC) analyzers pipeline market are Access Bio Inc., Acousort AB, Acrongenomics Inc. (Inactive), AgPlus Diagnostics LTD, and Akonni Biosystems Inc., among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Point of Care (POC) Analyzers reports