Remote Patient Monitoring – Pipeline Products by Stage of Development 36
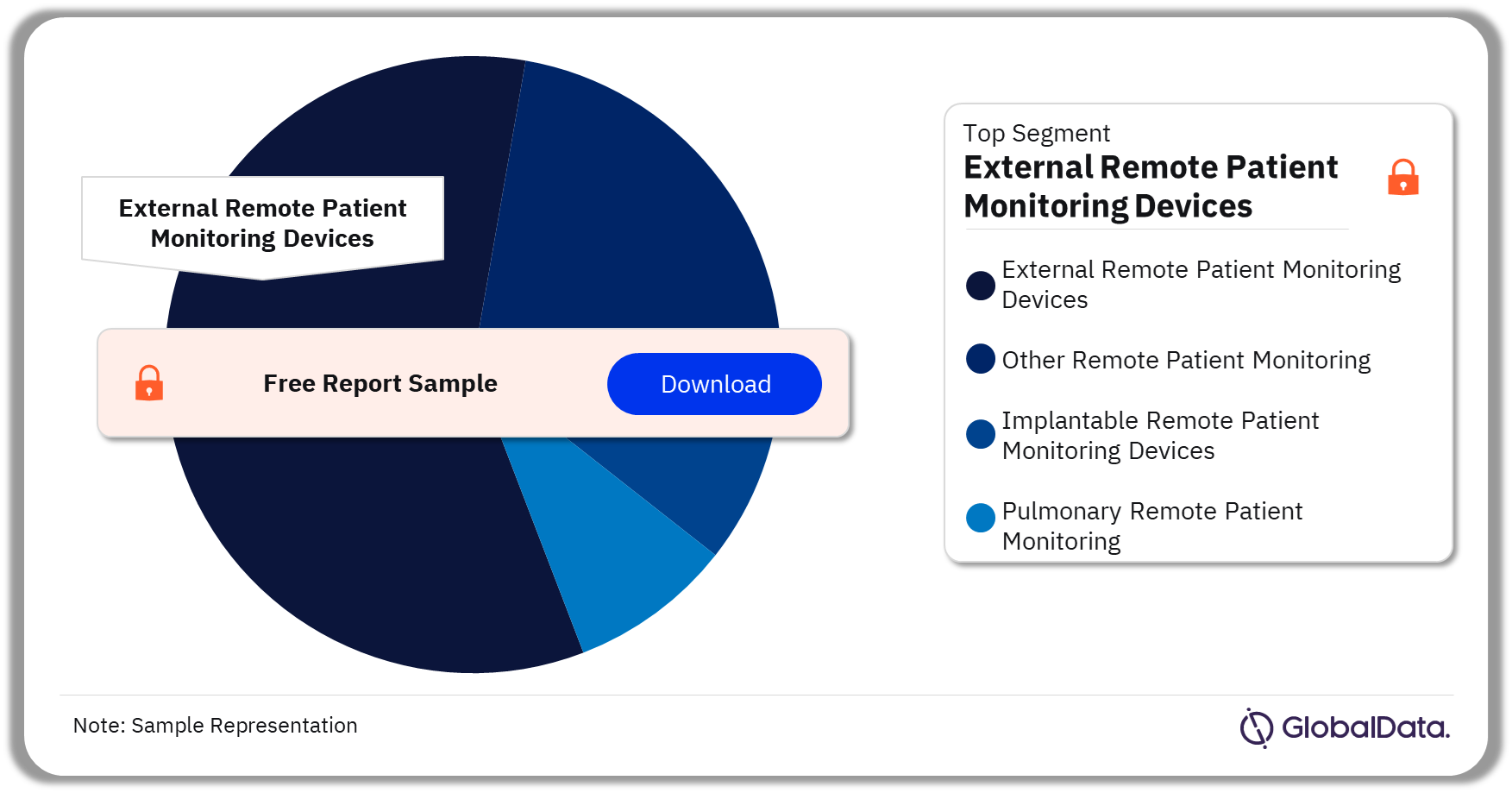
Remote Patient Monitoring – Pipeline Products by Segment 37
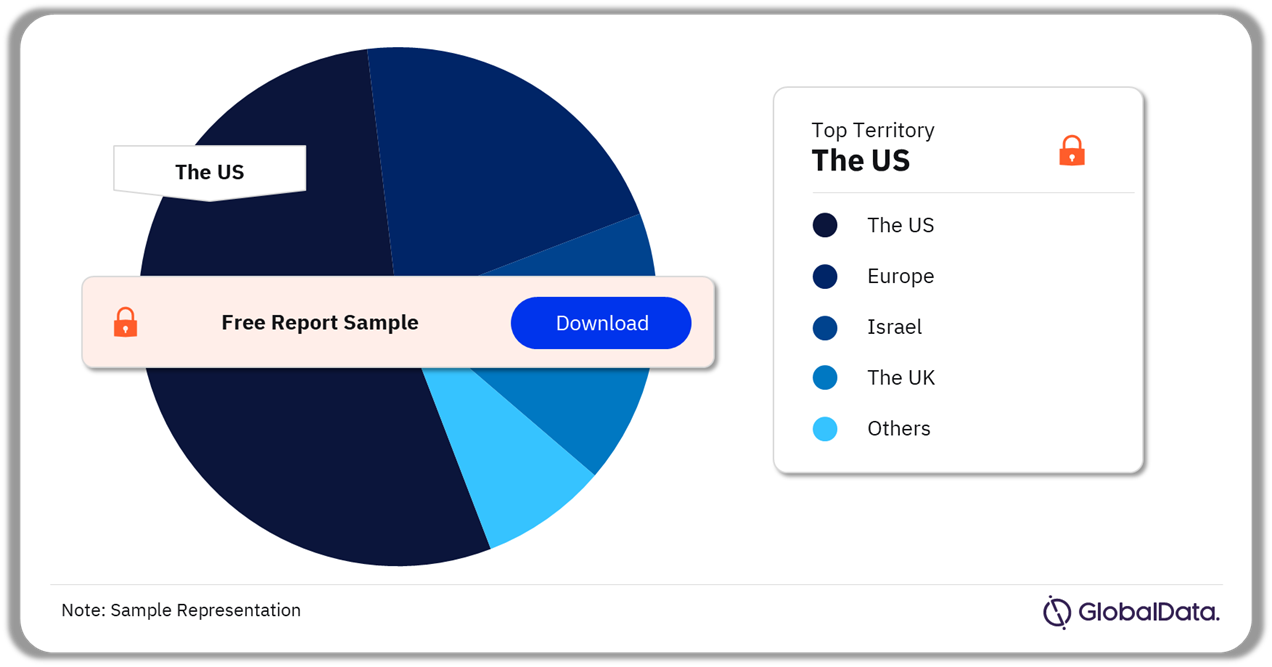
Remote Patient Monitoring – Pipeline Products by Territory 38
Remote Patient Monitoring – Pipeline Products by Regulatory Path 39
Remote Patient Monitoring – Pipeline Products by Estimated Approval Date 40
Remote Patient Monitoring – Ongoing Clinical Trials 41
Remote Patient Monitoring Companies – Pipeline Products by Stage of Development 42
Remote Patient Monitoring – Pipeline Products by Stage of Development 51
Abmi S.A. Pipeline Products & Ongoing Clinical Trials Overview 59
Patient Monitoring System – Stroke – Product Status 59
Patient Monitoring System – Stroke – Product Description 59
Active4D Inc Pipeline Products & Ongoing Clinical Trials Overview 60
Wearable Sensor Device – Product Status 60
Wearable Sensor Device – Product Description 60
ActiveCare Inc Pipeline Products & Ongoing Clinical Trials Overview 61
ActiveOne+ – Product Status 61
ActiveOne+ – Product Description 61
Advanced Remote Monitoring LLC Pipeline Products & Ongoing Clinical Trials Overview 62
armtrackr CCM+ – Product Status 62
armtrackr CCM+ – Product Description 62
Agali Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview 63
VigilCare – Product Status 63
VigilCare – Product Description 63
AirStrip Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 64
Airstrip mHealth Remote Care Information System – Product Status 64
Airstrip mHealth Remote Care Information System – Product Description 64
AISENS Sp zoo Pipeline Products & Ongoing Clinical Trials Overview 65
Fitnessyo – Product Status 65
Fitnessyo – Product Description 65
OrthyoPro – Product Status 66
OrthyoPro – Product Description 66
Alio Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 67
Alio SmartPatch – Product Status 67
Alio SmartPatch – Product Description 67
Allm Inc Pipeline Products & Ongoing Clinical Trials Overview 68
Smartphone App – Vital Sign Measurement – Product Status 68
Smartphone App – Vital Sign Measurement – Product Description 68
ALR Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 69
Health-e-Connect System – Blood Pressure Meter – Product Status 69
Health-e-Connect System – Blood Pressure Meter – Product Description 70
Health-e-Connect System – Body Composition Monitor – Product Status 70
Health-e-Connect System – Body Composition Monitor – Product Description 70
Health-e-Connect System – Electrocardiogram – Product Status 71
Health-e-Connect System – Electrocardiogram – Product Description 71
Health-e-Connect System – Peak Flow Meter – Product Status 71
Health-e-Connect System – Peak Flow Meter – Product Description 72
Health-e-Connect System – Pulse Oximeter – Product Status 72
Health-e-Connect System – Pulse Oximeter – Product Description 72
Health-e-Connect System – Respiratory Health Management – Product Status 73
Health-e-Connect System – Respiratory Health Management – Product Description 73
Amissa Inc Pipeline Products & Ongoing Clinical Trials Overview 74
Caregiver App – Product Status 74
Caregiver App – Product Description 74
Anglia Ruskin University Pipeline Products & Ongoing Clinical Trials Overview 75
Wearable Sensor – Respiratory Monitoring – Product Status 75
Wearable Sensor – Respiratory Monitoring – Product Description 75
Apogee Technology Inc Pipeline Products & Ongoing Clinical Trials Overview 76
IntellaPAL – Product Status 76
IntellaPAL – Product Description 76
Apple Inc Pipeline Products & Ongoing Clinical Trials Overview 77
Apple Watch – Glucose Monitoring – Product Status 77
Apple Watch – Glucose Monitoring – Product Description 77
Apptomics Inc Pipeline Products & Ongoing Clinical Trials Overview 78
E.V.A – Product Status 78
E.V.A – Product Description 78
ARC Devices Ltd. Pipeline Products & Ongoing Clinical Trials Overview 79
MedTemp RPM – Product Status 79
MedTemp RPM – Product Description 79
Arche Healthcare LLC Pipeline Products & Ongoing Clinical Trials Overview 80
Next Generation TempStat – Product Status 80
Next Generation TempStat – Product Description 80
Arcturis Data Ltd Pipeline Products & Ongoing Clinical Trials Overview 81
EDGE – Product Status 81
EDGE – Product Description 81
Support-HF – Product Status 82
Support-HF – Product Description 82
Arthronica Ltd Pipeline Products & Ongoing Clinical Trials Overview 83
Patient-Administered Disease Monitoring Device – Product Status 83
Patient-Administered Disease Monitoring Device – Product Description 83
Aseptika Ltd Pipeline Products & Ongoing Clinical Trials Overview 84
BuddyWOTCH – Product Status 84
BuddyWOTCH – Product Description 84
Aster Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 85
Activlink Insole – Product Status 85
Activlink Insole – Product Description 85
ATLASense Biomed Ltd Pipeline Products & Ongoing Clinical Trials Overview 86
PolyMonitor – Product Status 86
PolyMonitor – Product Description 86
Autonomous Healthcare Inc Pipeline Products & Ongoing Clinical Trials Overview 87
Noncontact Remote Monitoring Device – Opioid-Induced Respiratory Depression – Product Status 87
Noncontact Remote Monitoring Device – Opioid-Induced Respiratory Depression – Product Description 87
Avicena LLC Pipeline Products & Ongoing Clinical Trials Overview 88
Vivio Device – Product Status 88
Vivio Device – Product Description 88
Babyscripts Inc Pipeline Products & Ongoing Clinical Trials Overview 89
Postpartum Hypertension Module – Product Status 89
Postpartum Hypertension Module – Product Description 89
Postpartum Module – Product Status 90
Postpartum Module – Product Description 90
Prenatal Hypertension RPM Solution – Product Status 90
Prenatal Hypertension RPM Solution – Product Description 90
Barron Associates, Inc. Pipeline Products & Ongoing Clinical Trials Overview 91
FallCall System – Product Status 91
FallCall System – Product Description 91
Remote Oxygen Adherence Monitor System – Product Status 92
Remote Oxygen Adherence Monitor System – Product Description 92
TELEHOME System – Product Status 92
TELEHOME System – Product Description 93
VALOR System – Product Status 93
VALOR System – Product Description 93
Bender Tech LLC Pipeline Products & Ongoing Clinical Trials Overview 94
Heart Failure Monitoring Device – Product Status 94
Heart Failure Monitoring Device – Product Description 94
Berkshire Biomedical Corp Pipeline Products & Ongoing Clinical Trials Overview 95
Computerized Oral Prescription Administration System – Product Status 95
Computerized Oral Prescription Administration System – Product Description 96
Better Sense BV Pipeline Products & Ongoing Clinical Trials Overview 97
Ambulatory Drug Delivery IoT System – Product Status 97
Ambulatory Drug Delivery IoT System – Product Description 97
Bharat Electronics Ltd Pipeline Products & Ongoing Clinical Trials Overview 98
Remote Patient Monitoring Device – COVID-19 – Product Status 98
Remote Patient Monitoring Device – COVID-19 – Product Description 98
BioSensics LLC Pipeline Products & Ongoing Clinical Trials Overview 99
Care4AD – Product Status 99
Care4AD – Product Description 100
HDWear – Product Status 100
HDWear – Product Description 100
MGWear – Product Status 101
MGWear – Product Description 101
PAMSys ULM – Product Status 101
PAMSys ULM – Product Description 102
PAMSys+ – Product Status 102
PAMSys+ – Product Description 102
Remote Monitoring Tool – Frontotemporal Lobar Degeneration Syndrome – Product Status 103
Remote Monitoring Tool – Frontotemporal Lobar Degeneration Syndrome – Product Description 103
StrokeWear – Product Status 103
StrokeWear – Product Description 104
Tele-CF – Product Status 104
Tele-CF – Product Description 104
BioSensics LLC – Ongoing Clinical Trials Overview 105
StrokeWear – A Novel Wrist Wearable Sensor System to Promote Hemiparetic Upper Extremity Use in Home Daily Life of Chronic Stroke Survivors 106
Care4AD – A Comprehensive Care Coordination and Management Platform for Alzheimer’s Disease and Related Dementia (Care4AD) 107
Tele-CF – Tele-CF: A Practical Platform for Remote Monitoring of Cognitive Frailty 108
PAMSys ULM – Monitoring Biomarker for Detecting Change in Physical Activity and Limb Function in Inclusion Body Myositis Over Time 109
Biotricity Inc Pipeline Products & Ongoing Clinical Trials Overview 110
Sleep Apnea Device – Product Status 110
Sleep Apnea Device – Product Description 110
Bluedrop Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 111
Diabetic Foot Ulcer Device – Product Status 111
Diabetic Foot Ulcer Device – Product Description 111
Bluedrop Medical Ltd – Ongoing Clinical Trials Overview 112
Diabetic Foot Ulcer Device – Remote Use of Thermovisual Monitoring to Reduce the Rate of Re-Ulceration in Patients at Risk of Recurrent Diabetic Foot Ulcers 113
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 114
Latitude Vision – Product Status 114
Latitude Vision – Product Description 114
MultiSENSE Patient Management System – Product Status 115
MultiSENSE Patient Management System – Product Description 115
Next Generation Programmer – Product Status 115
Next Generation Programmer – Product Description 115
Brain Tunnelgenix Technologies Corp Pipeline Products & Ongoing Clinical Trials Overview 116
BTT Wireless Device – Product Status 116
BTT Wireless Device – Product Description 116
BrightOutcome Inc. Pipeline Products & Ongoing Clinical Trials Overview 117
SymptomCareAnywhere (SCA) System – Product Status 117
SymptomCareAnywhere (SCA) System – Product Description 117
Brilliant & Co Pipeline Products & Ongoing Clinical Trials Overview 118
Dorazee – Product Status 118
Dorazee – Product Description 118
Callyope Pipeline Products & Ongoing Clinical Trials Overview 119
Speech-Based Remote Patient Monitoring Tool – Product Status 119
Speech-Based Remote Patient Monitoring Tool – Product Description 119
CardiacSense Ltd Pipeline Products & Ongoing Clinical Trials Overview 120
CardiacSense – CS3 – Product Status 120
CardiacSense – CS3 – Product Description 121
CardiacSense – CS5 – Product Status 121
CardiacSense – CS5 – Product Description 121
CardiacSense – CS6 – Product Status 122
CardiacSense – CS6 – Product Description 122
CardiacSense – CS7 – Product Status 122
CardiacSense – CS7 – Product Description 123
CardiacSense – CS8 – Product Status 123
CardiacSense – CS8 – Product Description 123
CardiacSense – CSF4 – Product Status 124
CardiacSense – CSF4 – Product Description 124
CardiacSense – CSF5 – Product Status 124
CardiacSense – CSF5 – Product Description 125
CardiacSense – CSF6 – Product Status 125
CardiacSense – CSF6 – Product Description 125
CardiacSense – Remote ECG Monitoring – Product Status 126
CardiacSense – Remote ECG Monitoring – Product Description 126
CSF-3 – Product Status 126
CSF-3 – Product Description 127
CardiacSense Ltd – Ongoing Clinical Trials Overview 128
CSF-3 – Anonymized Data Collection from the CardiacSense1 and Other Modalities for the Purpose of Developing a System for Monitoring of Respiratory Rate 129
CardieX Ltd Pipeline Products & Ongoing Clinical Trials Overview 130
PPG Sensor – Product Status 130
PPG Sensor – Product Description 130
CardioComm Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 131
Multiple Biosign Monitoring Device – Product Status 131
Multiple Biosign Monitoring Device – Product Description 131
CardioSounds LLC Pipeline Products & Ongoing Clinical Trials Overview 132
CardioSensor – Product Status 132
CardioSensor – Product Description 132
CardioStory Inc Pipeline Products & Ongoing Clinical Trials Overview 133
NIFP Device – Heart Failure – Product Status 133
NIFP Device – Heart Failure – Product Description 133
CARI Health Inc Pipeline Products & Ongoing Clinical Trials Overview 134
BioMote – Product Status 134
BioMote – Product Description 134
CARI Health Inc – Ongoing Clinical Trials Overview 135
BioMote – Assessing Dose Taken in Opioid Use Disordered Patients with an Electrochemical Sensor 136
BioMote – Assessment of Methadone Dose Taken Using Electrochemistry 136
CereVu Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 137
Remote COVID-19 Sensor – Product Status 137
Remote COVID-19 Sensor – Product Description 137
CFD Research Corp Pipeline Products & Ongoing Clinical Trials Overview 138
Vestibular Rehabilitation App – Product Status 138
Vestibular Rehabilitation App – Product Description 138
CFD Research Corp – Ongoing Clinical Trials Overview 139
Vestibular Rehabilitation App – Evaluation of an App for Individualized Vestibular Rehabilitation for Elderly With Self-Management and Gaming Elements 140
Chronolife Pipeline Products & Ongoing Clinical Trials Overview 141
KeeSense – Product Status 141
KeeSense – Product Description 141
KeeSense – Joint Diseases – Product Status 142
KeeSense – Joint Diseases – Product Description 142
Chronolife – Ongoing Clinical Trials Overview 143
KeeSense – Study to Demonstrate the Role of Keesense, a Remote Patient Monitoring Device in Enhancing the Oncology Patient’s Care Pathway 144
Cloud DX Inc. Pipeline Products & Ongoing Clinical Trials Overview 145
Vitaliti Wearable Health Monitor – Product Status 145
Vitaliti Wearable Health Monitor – Product Description 145
Cnoga Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 146
Smart Wearable Capsule – Product Status 146
Smart Wearable Capsule – Product Description 146
Cognizant Technology Solutions Corp Pipeline Products & Ongoing Clinical Trials Overview 147
Virtual Healthcare Solution – Product Status 147
Virtual Healthcare Solution – Product Description 147
ConnEQt Pipeline Products & Ongoing Clinical Trials Overview 148
Conneqt Band – Product Status 148
Conneqt Band – Product Description 148
Consensus Orthopedics Inc Pipeline Products & Ongoing Clinical Trials Overview 149
TracPatch – Product Status 149
TracPatch – Product Description 149
TracPatch ACL – Product Status 150
TracPatch ACL – Product Description 150
TracPatch Hip – Product Status 150
TracPatch Hip – Product Description 151
TracPatch Shoulder – Product Status 151
TracPatch Shoulder – Product Description 151
TracPatch Spine – Product Status 152
TracPatch Spine – Product Description 152
Consensus Orthopedics Inc – Ongoing Clinical Trials Overview 153
TracPatch – Evaluating the Efficacy of the TracPatch Wearable Technology on Patients Undergoing Total Knee Arthroplasty (TKA) 154
Coprata Inc Pipeline Products & Ongoing Clinical Trials Overview 155
Coprata Smart Sampling Toilet – Product Status 155
Coprata Smart Sampling Toilet – Product Description 155
Corsano Health BV Pipeline Products & Ongoing Clinical Trials Overview 156
CardioWatch 287-2 – Product Status 156
CardioWatch 287-2 – Product Description 156
Corsano Health BV – Ongoing Clinical Trials Overview 157
CardioWatch 287-2 – Remote Cardiac Monitoring by the Corsano CardioWatch 287-2 Evaluation Study 158
CorTronix Biomedical Advancement Technologies Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 159
CorTab Medical Telemetry System – Product Status 159
CorTab Medical Telemetry System – Product Description 159
CorView – Product Status 160
CorView – Product Description 160
Creative Action LLC Pipeline Products & Ongoing Clinical Trials Overview 161
Tardive Dyskinesia Detection Tool – Product Status 161
Tardive Dyskinesia Detection Tool – Product Description 161
Critical Signal Technologies Pipeline Products & Ongoing Clinical Trials Overview 162
WristClinic – Product Status 162
WristClinic – Product Description 162
Curasis LLC Pipeline Products & Ongoing Clinical Trials Overview 163
Tele-EaT Sensor – Product Status 163
Tele-EaT Sensor – Product Description 163
Current Health Ltd Pipeline Products & Ongoing Clinical Trials Overview 164
AI-Based Algorithm – COVID-19 – Product Status 164
AI-Based Algorithm – COVID-19 – Product Description 165
AI-Powered RPM – CGM – Product Status 165
AI-Powered RPM – CGM – Product Description 166
CUSH Health Ltd Pipeline Products & Ongoing Clinical Trials Overview 167
CUSH Digital Patient Monitor – Product Status 167
CUSH Digital Patient Monitor – Product Description 167
Cydelic Inc Pipeline Products & Ongoing Clinical Trials Overview 168
Wearable Remote Monitoring Device – Product Status 168
Wearable Remote Monitoring Device – Product Description 168
Dawn Health AS Pipeline Products & Ongoing Clinical Trials Overview 169
Chronic Disease Software App – Product Status 169
Chronic Disease Software App – Product Description 169
Deep Breeze Ltd Pipeline Products & Ongoing Clinical Trials Overview 170
Breeze@home – Product Status 170
Breeze@home – Product Description 170
Diabetech LP Pipeline Products & Ongoing Clinical Trials Overview 171
GlucoDYNAMIX – Product Status 171
GlucoDYNAMIX – Product Description 171
Dianosic Pipeline Products & Ongoing Clinical Trials Overview 172
Connected Asymmetric Balloon – Product Status 172
Connected Asymmetric Balloon – Product Description 172
Difinity Solutions Pipeline Products & Ongoing Clinical Trials Overview 173
NeedSense – Product Status 173
NeedSense – Product Description 173
Eccrine Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 174
Non-Invasive Sweat Sensor – Product Status 174
Non-Invasive Sweat Sensor – Product Description 174
Non-Invasive Wearable Device – Cognitive Status – Product Status 175
Non-Invasive Wearable Device – Cognitive Status – Product Description 175
Non-Invasive Wearable Device – Depression – Product Status 175
Non-Invasive Wearable Device – Depression – Product Description 176
Non-Invasive Wearable Device – Infection – Product Status 176
Non-Invasive Wearable Device – Infection – Product Description 176
Elpidatec Inc Pipeline Products & Ongoing Clinical Trials Overview 177
Elpida Software – Product Status 177
Elpida Software – Product Description 177
ELTA Systems Ltd Pipeline Products & Ongoing Clinical Trials Overview 178
Vital Sign Monitoring System – COVID-19 – Product Status 178
Vital Sign Monitoring System – COVID-19 – Product Description 178
Emerald Innovations Inc Pipeline Products & Ongoing Clinical Trials Overview 179
Emerald Touchless Sensor – Product Status 179
Emerald Touchless Sensor – Product Description 179
Emerald Innovations Inc – Ongoing Clinical Trials Overview 180
Emerald Touchless Sensor – Objective, Passive Assessment of LRRK2 Carriers 181
Entia Ltd Pipeline Products & Ongoing Clinical Trials Overview 182
Entia Liberty – Product Status 182
Entia Liberty – Product Description 182
Entia Ltd – Ongoing Clinical Trials Overview 183
Entia Liberty – Capillary-Venous Paired Data Collection 184
Entia Liberty – Entia Liberty: Home Study – Evaluation of Patient Preference for a Novel Full Blood Count Home Testing Device Versus Routine Venous Monitoring 184
Entia Liberty – HOme USability Evaluation 184
Epicore Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview 185
Sweat Sensor – Product Status 185
Sweat Sensor – Product Description 185
EpiWatch Inc Pipeline Products & Ongoing Clinical Trials Overview 186
Tonic-Clonic Epileptic Seizure Detection and Alerting Software – Product Status 186
Tonic-Clonic Epileptic Seizure Detection and Alerting Software – Product Description 186
Essence SmartCare Ltd Pipeline Products & Ongoing Clinical Trials Overview 187
Umbrella mPERS – Product Status 187
Umbrella mPERS – Product Description 187
EXODUS Anonymos Etaireia Pliroforikis Pipeline Products & Ongoing Clinical Trials Overview 188
WELMO System – Product Status 188
WELMO System – Product Description 188
Federal University of Espirito Santo Pipeline Products & Ongoing Clinical Trials Overview 189
Distress Detection Smart Pants – Product Status 189
Distress Detection Smart Pants – Product Description 189
Ford Research Centre Pipeline Products & Ongoing Clinical Trials Overview 190
In-Car Heart Monitor – Product Status 190
In-Car Heart Monitor – Product Description 190
Foundry Innovation & Research 1 Ltd Pipeline Products & Ongoing Clinical Trials Overview 191
FIRE1 System – Product Status 191
FIRE1 System – Product Description 191
Foundry Innovation & Research 1 Ltd – Ongoing Clinical Trials Overview 192
FIRE1 System – Early Feasibility Study of the FIRE1 System in Heart Failure Patients 193
FIRE1 System – First In Human Clinical Investigation of the FIRE1 System in Heart Failure Patients 193
Freescale Semiconductor, Ltd. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 194
Medical Kiosk – Product Status 194
Medical Kiosk – Product Description 194
Futura Mobile Health, LLC Pipeline Products & Ongoing Clinical Trials Overview 195
SmartScope System – Product Status 195
SmartScope System – Product Description 195
Gabi Smartcare SA Pipeline Products & Ongoing Clinical Trials Overview 196
Digital Diagnostic Wearable Device – Product Status 196
Digital Diagnostic Wearable Device – Product Description 196
Grapheal SAS Pipeline Products & Ongoing Clinical Trials Overview 197
WoundLAB – Product Status 197
WoundLAB – Product Description 197
Great Lakes NeuroTechnologies Inc Pipeline Products & Ongoing Clinical Trials Overview 198
Discern Candidacy And Evaluation System – Product Status 198
Discern Candidacy And Evaluation System – Product Description 198
Great Lakes NeuroTechnologies Inc – Ongoing Clinical Trials Overview 199
Discern Candidacy And Evaluation System – DiSCERN: Advanced PD Therapy Candidacy and Evaluation System – Advanced PD Data Collection 200
Grey Innovation Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 201
Osmetric – Product Status 201
Osmetric – Product Description 201
Guide Analytics Inc. Pipeline Products & Ongoing Clinical Trials Overview 202
Ankle Bracelet Monitor – Product Status 202
Ankle Bracelet Monitor – Product Description 202
Harvard Medical School Pipeline Products & Ongoing Clinical Trials Overview 203
Wearable Sensor Device – Product Status 203
Wearable Sensor Device – Product Description 203
HD Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 204
HealthyU – Product Status 204
HealthyU – Product Description 204
Heart Health, Inc. Pipeline Products & Ongoing Clinical Trials Overview 205
Heart Health Monitor – Product Status 205
Heart Health Monitor – Product Description 205
Heartfelt Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 206
Heartfelt Device – Product Status 206
Heartfelt Device – Product Description 206
Heartfelt Technologies Ltd – Ongoing Clinical Trials Overview 207
Heartfelt Device – MONITORED-HF: Remote Monitoring of Ambulatory Intravenous Diuretics in Heart Failure 208
Heroic-Faith Medical Science Corp Pipeline Products & Ongoing Clinical Trials Overview 209
Automatic Lung Sound Monitor – Product Status 209
Automatic Lung Sound Monitor – Product Description 209
Hoffmann-La Roche Inc Pipeline Products & Ongoing Clinical Trials Overview 210
Remote Monitoring System – Product Status 210
Remote Monitoring System – Product Description 210
Human Telemetry Data Management Services LLC Pipeline Products & Ongoing Clinical Trials Overview 211
Human Telemetry Device – Product Status 211
Human Telemetry Device – Product Description 211
Huxley Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 212
Wireless Sensor Patch – Heart Failure – Product Status 212
Wireless Sensor Patch – Heart Failure – Product Description 212
Wireless Sensor Patch – Obstructive Sleep Apnea – Product Status 213
Wireless Sensor Patch – Obstructive Sleep Apnea – Product Description 213
Hydrostasis Inc Pipeline Products & Ongoing Clinical Trials Overview 214
Wearable Hydration Monitoring System – Product Status 214
Wearable Hydration Monitoring System – Product Description 214
iBeat Inc Pipeline Products & Ongoing Clinical Trials Overview 215
iBeat Heart Watch – Product Status 215
iBeat Heart Watch – Product Description 215
Ibridge Medical, LLC Pipeline Products & Ongoing Clinical Trials Overview 216
iBridge Device – Product Status 216
iBridge Device – Product Description 216
IDION LLC Pipeline Products & Ongoing Clinical Trials Overview 217
iVitalShield – Product Status 217
iVitalShield – Product Description 217
Ikinova Pipeline Products & Ongoing Clinical Trials Overview 218
Inova CARE – Product Status 218
Inova CARE – Product Description 218
Inova MED – Product Status 219
Inova MED – Product Description 219
IMP Scandinavia Aps Pipeline Products & Ongoing Clinical Trials Overview 220
Smart Patch – Version 2.0 – Product Status 220
Smart Patch – Version 2.0 – Product Description 220
Implantica CE & Production Ltd Pipeline Products & Ongoing Clinical Trials Overview 221
AneurysmControl – Product Status 221
AneurysmControl – Product Description 221
InCardia Inc Pipeline Products & Ongoing Clinical Trials Overview 222
CorWatch – Product Status 222
CorWatch – Product Description 222
InCardia Inc – Ongoing Clinical Trials Overview 223
CorWatch – Comparison of the InCardia Venous Pressure Diagnostic Technology CorWatch with Swan Ganz Catheter Measurements in the Cardiac Catheterization Laboratory and Cardiac Intensive Care Unit 224
Indian Institute of Technology Hyderabad Pipeline Products & Ongoing Clinical Trials Overview 225
Remote Monitoring Device – COVID-19 – Product Status 225
Remote Monitoring Device – COVID-19 – Product Description 225
InfoBionic Inc Pipeline Products & Ongoing Clinical Trials Overview 226
MoMe ARC – Product Status 226
MoMe ARC – Product Description 226
MoMe Gateway – Product Status 227
MoMe Gateway – Product Description 227
MoMe Now – Product Status 227
MoMe Now – Product Description 228
InjectSense Inc Pipeline Products & Ongoing Clinical Trials Overview 229
iOP-Connect System – Product Status 229
iOP-Connect System – Product Description 229
Innovative Design Labs Inc Pipeline Products & Ongoing Clinical Trials Overview 230
Sleep Quality Monitor – Product Status 230
Sleep Quality Monitor – Product Description 230
Innovative Design Labs Inc – Ongoing Clinical Trials Overview 231
Sleep Quality Monitor – Automated, Assistive, Non-Contact Sleep Quality Monitor for Individuals with Alzheimer’s Disease 232
Inspire Living, Inc. Pipeline Products & Ongoing Clinical Trials Overview 233
Inspire Monitor – Product Status 233
Inspire Monitor – Product Description 233
Institute for Infocomm Research Pipeline Products & Ongoing Clinical Trials Overview 234
Human Activity Modelling System – Product Status 234
Human Activity Modelling System – Product Description 234
Intelligent Implants Pipeline Products & Ongoing Clinical Trials Overview 235
SmartFuse System – Product Status 235
SmartFuse System – Product Description 235
Iron Bow Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 236
CLINiC Device Integrated Telehealth Software – Product Status 236
CLINiC Device Integrated Telehealth Software – Product Description 236
Itrachealth Corp Pipeline Products & Ongoing Clinical Trials Overview 237
eSSIST Remote Caregiver Smart Phone App – Product Status 237
eSSIST Remote Caregiver Smart Phone App – Product Description 237
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 238
Smart Ankle Bracelet – Product Status 238
Smart Ankle Bracelet – Product Description 238
JointMetrix Medical, LLC Pipeline Products & Ongoing Clinical Trials Overview 239
Joint Monitoring System – Product Status 239
Joint Monitoring System – Product Description 239
JuneBrain, LLC Pipeline Products & Ongoing Clinical Trials Overview 240
Neuro-i SS-OCT – Product Status 240
Neuro-i SS-OCT – Product Description 240
Kaiku Health Oy Pipeline Products & Ongoing Clinical Trials Overview 241
Digital Patient Monitoring Algorithm – Melanoma – Product Status 241
Digital Patient Monitoring Algorithm – Melanoma – Product Description 241
Keck Medicine of USC Pipeline Products & Ongoing Clinical Trials Overview 242
Smart Boot – Product Status 242
Smart Boot – Product Description 242
Keck Medicine of USC – Ongoing Clinical Trials Overview 243
Smart Boot – Study to Evaluate the Efficacy of Smart Offloading Boot System for Remote Patient Monitoring in Diabetic Foot Ulcer Patients 244
Kiddo Health Inc Pipeline Products & Ongoing Clinical Trials Overview 245
Kiddo – Product Status 245
Kiddo – Product Description 245
Kimberly-Clark Corp Pipeline Products & Ongoing Clinical Trials Overview 246
Heat Stress Sensor – Product Status 246
Heat Stress Sensor – Product Description 246
KK Women’s and Children’s Hospital Pipeline Products & Ongoing Clinical Trials Overview 247
VPIA Delivery System – Product Status 247
VPIA Delivery System – Product Description 247
Koronis Biomedical Technologies Corporation Pipeline Products & Ongoing Clinical Trials Overview 248
Home Monitoring System – Cystic Fibrosis – Product Status 248
Home Monitoring System – Cystic Fibrosis – Product Description 248
K-Tree Srl Pipeline Products & Ongoing Clinical Trials Overview 249
CB-o Monitoring System – Product Status 249
CB-o Monitoring System – Product Description 249
Kyocera Corp Pipeline Products & Ongoing Clinical Trials Overview 250
Contactless Intelligent Millimeter-Wave Sensing System – Product Status 250
Contactless Intelligent Millimeter-Wave Sensing System – Product Description 251
Wearable Headset – Product Status 251
Wearable Headset – Product Description 251
Wireless Headset – Product Status 252
Wireless Headset – Product Description 252
Leaf Space Srl Pipeline Products & Ongoing Clinical Trials Overview 253
CARES Monitoring System – Product Status 253
CARES Monitoring System – Product Description 253
LiberDi Ltd Pipeline Products & Ongoing Clinical Trials Overview 254
Intelligent Dialysis Assistant (IDA) – Product Status 254
Intelligent Dialysis Assistant (IDA) – Product Description 254
LiberDi Ltd – Ongoing Clinical Trials Overview 255
Intelligent Dialysis Assistant (IDA) – A Prospective, Open-label, Cross-over, Multi-center Pilot Study to Evaluate the Safety, Feasibility and Usability of the IDA in Subjects Suffering From a Stage 5 Kidney Disease and Who Are Treated With Peritoneal Dialysis 256
Intelligent Dialysis Assistant (IDA) – A Single Arm, Prospective, Open Label, Cross-over, Multi-center Study to Evaluate the Safety, Feasibility and Usability of the Intelligent Dialysis Assistant (IDA) 256
Lifelong Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview 257
Step Activity Monitor – Product Status 257
Step Activity Monitor – Product Description 257
LifeSignals Pipeline Products & Ongoing Clinical Trials Overview 258
Biosensor Patch 2A – Product Status 258
Biosensor Patch 2A – Product Description 258
Louis Stokes Cleveland VA Medical Center Pipeline Products & Ongoing Clinical Trials Overview 259
Blood Pressure Sensor Implant – Product Status 259
Blood Pressure Sensor Implant – Product Description 259
Ludwig-Maximilians-University Munich Pipeline Products & Ongoing Clinical Trials Overview 260
IntelliTuM – Product Status 260
IntelliTuM – Product Description 260
lvlAlpha Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 261
HadEON and ArchEON Wearable Trauma Monitor/Communicator – Product Status 261
HadEON and ArchEON Wearable Trauma Monitor/Communicator – Product Description 261
Massachusetts General Hospital Pipeline Products & Ongoing Clinical Trials Overview 262
Remote Electronic Patient Monitoring Device – Product Status 262
Remote Electronic Patient Monitoring Device – Product Description 262
MC10 Inc Pipeline Products & Ongoing Clinical Trials Overview 263
NIMBLE Patch – Parkinson’s Disease – Product Status 263
NIMBLE Patch – Parkinson’s Disease – Product Description 263
MDDRIVEN LLC Pipeline Products & Ongoing Clinical Trials Overview 264
Lung and Cardiac Assessment Device – Product Status 264
Lung and Cardiac Assessment Device – Product Description 264
Medentum Innovations Inc Pipeline Products & Ongoing Clinical Trials Overview 265
COPD Management Home-Use Device – Product Status 265
COPD Management Home-Use Device – Product Description 265
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 266
Next Gen Vital Sync – Product Status 266
Next Gen Vital Sync – Product Description 266
Pelvic Remote Case Support Device – Product Status 267
Pelvic Remote Case Support Device – Product Description 267
MedWand Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 268
MedWand – Electrocardiogram – Product Status 268
MedWand – Electrocardiogram – Product Description 268
Michigan State University Pipeline Products & Ongoing Clinical Trials Overview 269
Wearable Sensor – Product Status 269
Wearable Sensor – Product Description 269
MicroPort CRM SA Pipeline Products & Ongoing Clinical Trials Overview 270
SmartView Connect Bluetooth Home Monitor – Product Status 270
SmartView Connect Bluetooth Home Monitor – Product Description 270
Microtech Group Ltd Pipeline Products & Ongoing Clinica
![]()