Reproductive Health Devices Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Reproductive Health Devices Pipeline Market Report Overview
Endometrial ablation devices, female sterilization devices, reversible contraceptive devices, and assisted reproductive technology devices are the main categories of reproductive health devices. The reproductive health devices pipeline report provides comprehensive information about the main products with their comparative segment-wise analysis at various stages of development.
The report also covers the regional and country-wise reproductive health devices clinical trials outlook. The regulatory paths followed for conducting the reproductive health devices pipeline research are also highlighted in the report. Leading companies involved in the reproductive health devices pipeline development are also enlisted in this research report to aid clients in effective decision-making.
| Key Segments | · Reversible Contraceptive Devices
· Intrauterine Devices (IUDs) · Copper IUDs · Hormonal IUDs · Endometrial Ablation Devices |
| Key Territories | · The US
· Europe · Japan · Mexico · New Zealand |
| Key Regulatory Paths | · 510(k)
· CE · PMA · TGA · Ninsho |
| Leading Companies | · AdneXXA LLC
· Agile Therapeutics Inc · AltaScience Limited · Bayer AG · Bioceptive Inc · Biorings LLC |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Reproductive Health Devices Pipeline Market by Segments
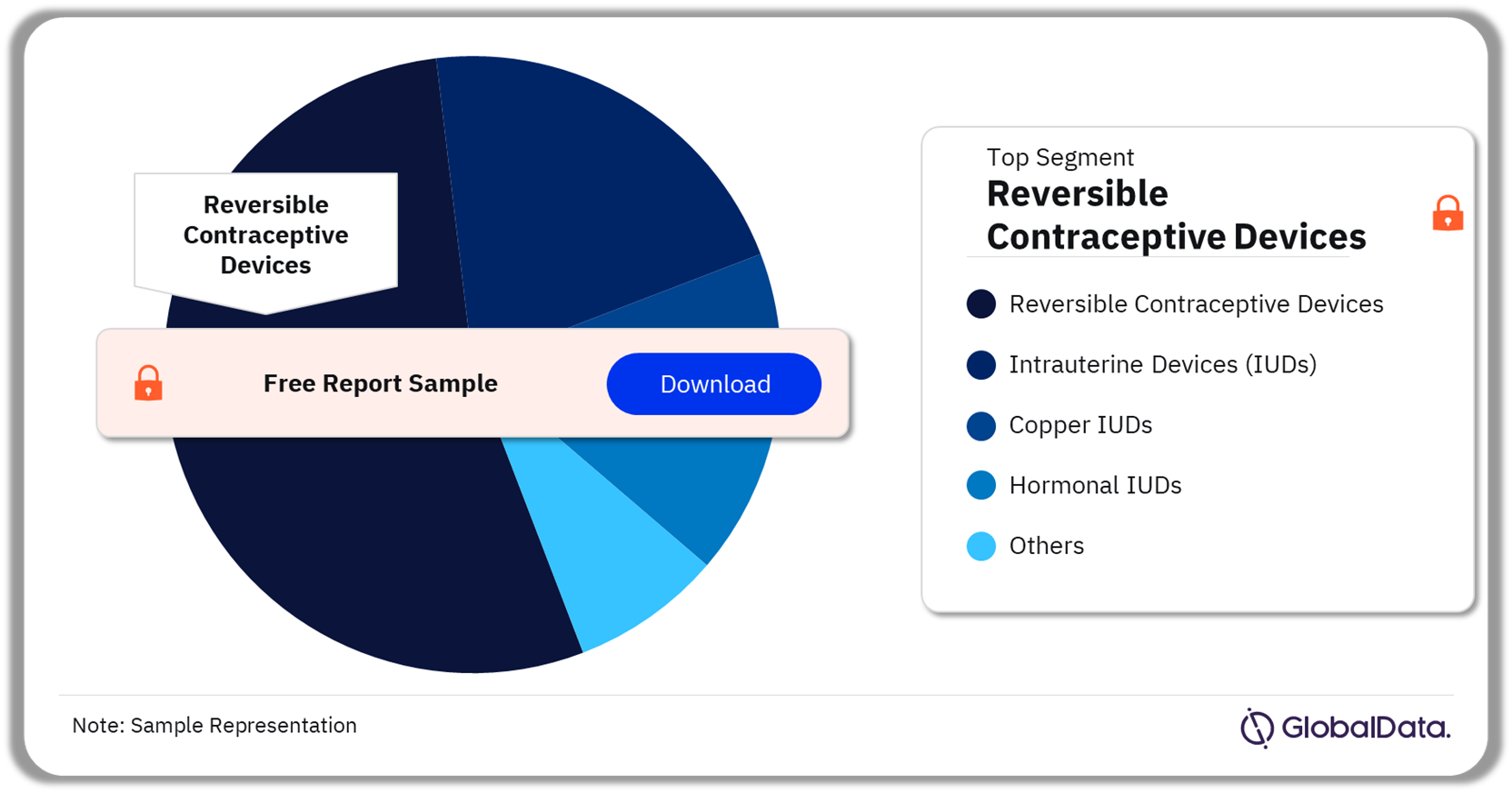
The key segments in the reproductive health devices pipeline market are reversible contraceptive devices, intrauterine devices (IUDs), copper IUDs, hormonal IUDs, and endometrial ablation devices among others. As of February 2024, the reversible contraceptive devices segment was the leading segment accounting for the highest number of products in the pipeline.
Reproductive Health Devices Pipeline Market Analysis by Segments, 2024 (%)
Buy the Full Report for Reproductive Health Devices Pipeline Market Segment Outlook
Reproductive Health Devices Pipeline Market Segmentation by Territories
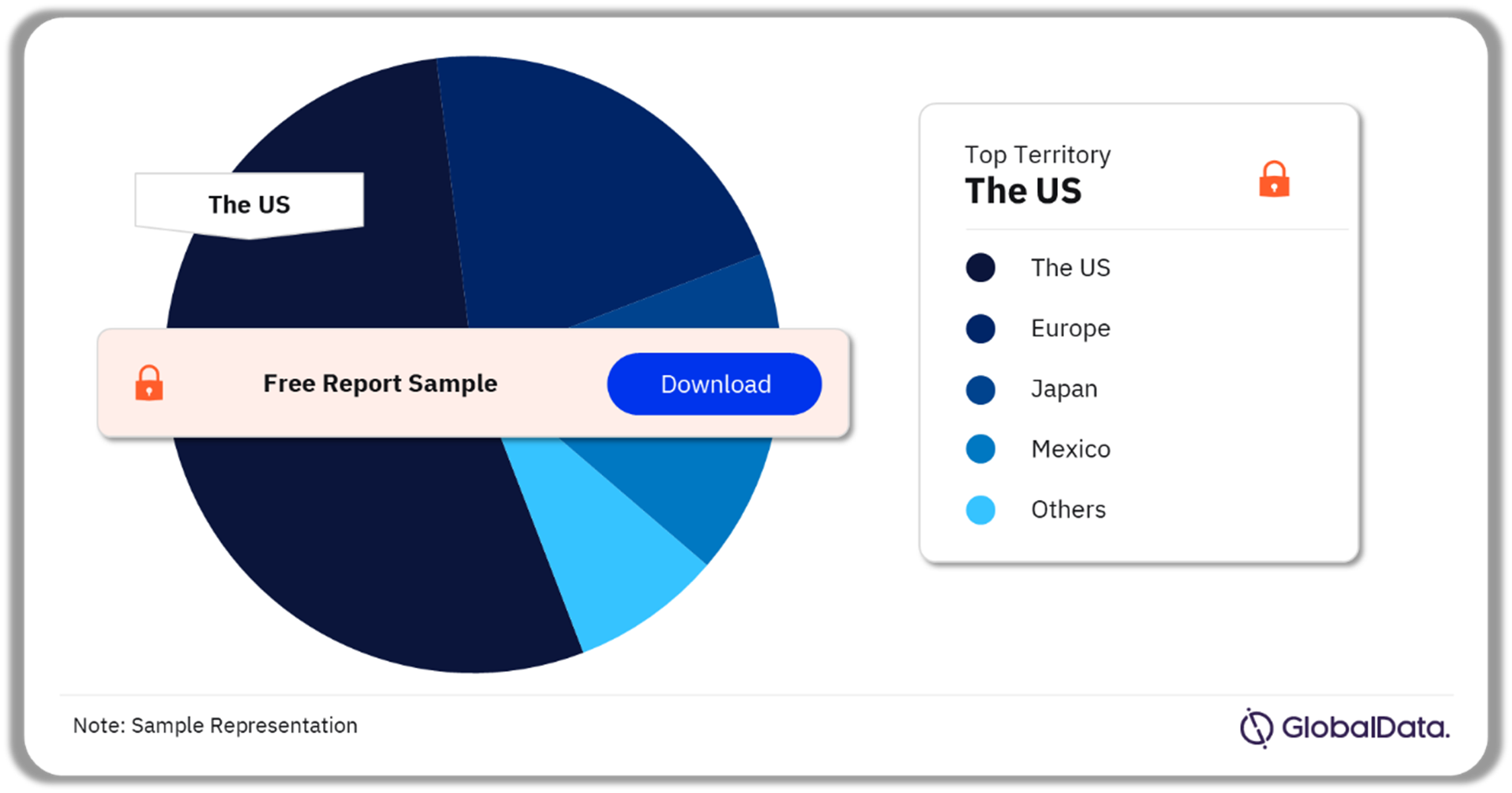
A few of the key territories for reproductive health devices pipeline products are the US, Europe, Japan, Mexico, and New Zealand among others. As of February 2024, the US accounted for the highest number of pipeline products.
Reproductive Health Devices Pipeline Market Analysis by Territories, 2024 (%)
Buy the Full Report for Territorial Reproductive Health Devices Pipeline Market Insights
Reproductive Health Devices Pipeline Market Segmentation by Regulatory Paths
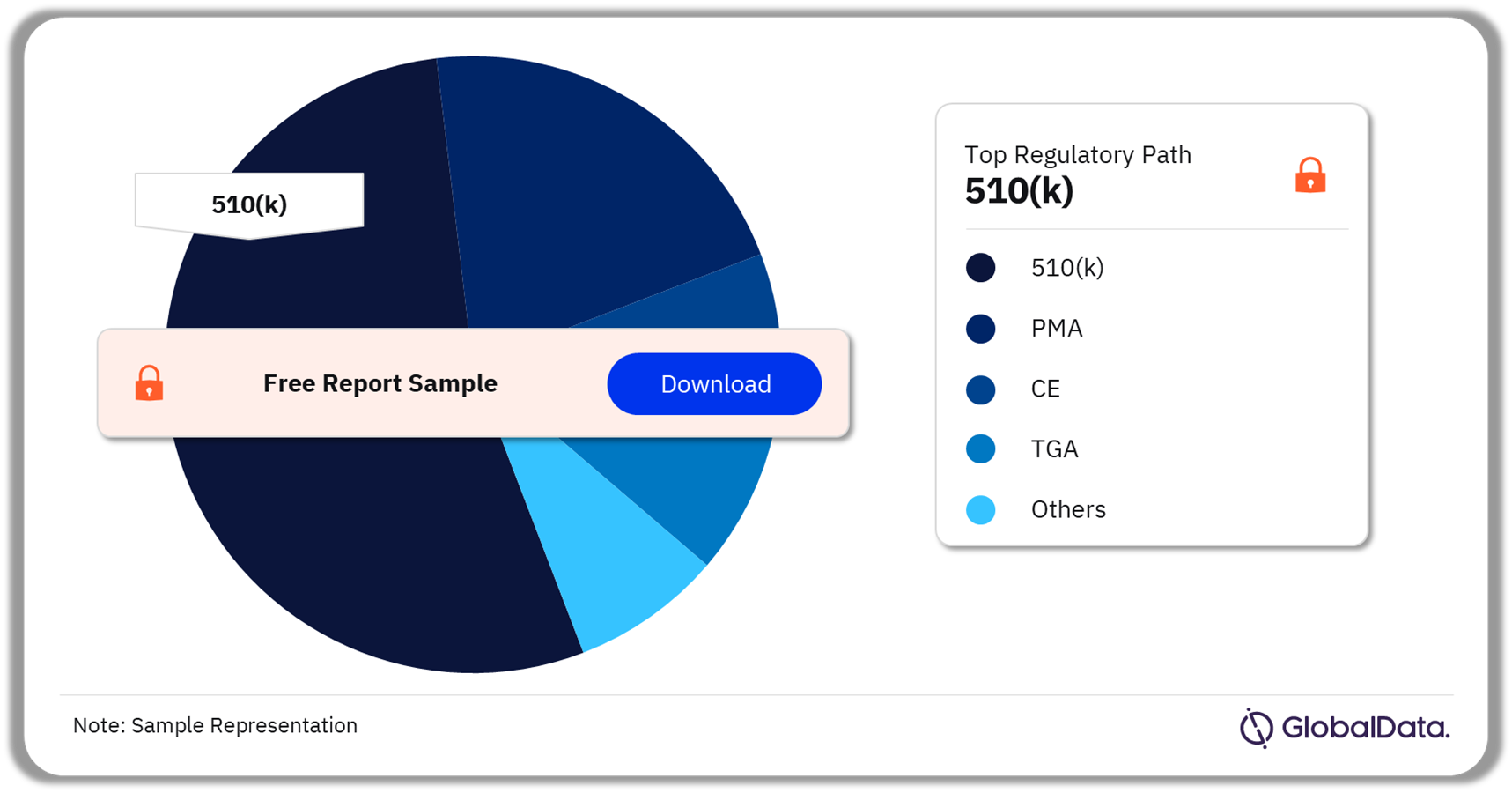
A few of the key regulatory paths followed by the reproductive health devices pipeline market are 510(k), PMA, CE, TGA, and NMPA. As of February 2024, 510(k) was the most followed pathway for pipeline products in the market.
Reproductive Health Devices Pipeline Market Analysis by Regulatory Paths, 2024 (%)
Buy the Full Report for More Regulatory Path Insights into the Reproductive Health Devices Pipeline Market
Reproductive Health Devices Pipeline Market – Competitive Landscape
A few of the key companies associated with the reproductive health devices pipeline market are AdneXXA LLC, Agile Therapeutics Inc, AltaScience Limited, Bayer AG, Bioceptive Inc, and Biorings LLC among others.
Agile Therapeutics Inc (Agile): Agile is headquartered in Princeton, New Jersey, the US. The company develops and commercializes prescription contraceptive products for women. The company offers contraceptive patch containing the active ingredients levonorgestrel and ethinyl estradiol and progestin-only birth control patch. Its pipeline product candidate includes Twirla also known as AG200-15, is a prescription combination hormonal contraceptive patch.
Bayer AG: Bayer is headquartered in Leverkusen, North Rhine-Westphalia, Germany. The company is engaged in the discovery, development, manufacturing, and commercialization of products for human health, and agriculture. It provides medicines for cardiovascular diseases, women’s health, cancer, hematology, ophthalmology, and other indications.
Reproductive Health Devices Pipeline Market Analysis by Companies, 2024
Buy the Full Report for More Company Insights into the Reproductive Health Devices Pipeline Market
Download a Free Report Sample
Segments Covered in the Report
Reproductive Health Devices Pipeline Market Segments Outlook
- Reversible Contraceptive Devices
- Intrauterine Devices (IUDs)
- Copper IUDs
- Hormonal IUDs
- Endometrial Ablation Devices
Reproductive Health Devices Pipeline Market Territories Outlook
- The US
- Europe
- Japan
- Mexico
- New Zealand
Reproductive Health Devices Pipeline Market Regulatory Paths Outlook
- 510(k)
- PMA
- CE
- TGA
- NMPA
Scope
This report provides:
- Extensive coverage of the reproductive health devices under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of reproductive health devices and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of reproductive health devices under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out an in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Ablatus Therapeutics Ltd
AdneXXA LLC
Agile Therapeutics Inc
AltaScience Limited
Alydia Health Inc
Autoivf Inc
Bayer AG
Bioceptive Inc
Biorings LLC
BioTex Inc
Ceragenix Pharmaceuticals, Inc. (Inactive)
Contrel Europe NV
Dare Bioscience Inc
Dartmouth College
Emblation Ltd
EVE Medical Systems Ltd
Evestra Inc
Evofem Biosciences Inc
Femasys Inc
FemSuite LLC
Ghent University
Healthcare Enterprise Group PLC (Inactive)
Hemosquid SAS
Hera Health Solutions
HLL Lifecare Ltd
Idoman Teoranta
Impres Medical, Inc.
INVO Bioscience Inc
Japanese Organization for Medical Device Development Inc
Johns Hopkins University
Keratin Biosciences Inc
Laboratoire HRA Pharma SAS
Lubrizol Life Science Health
May Health
Memorial Sloan Kettering Cancer Center
Memphasys Ltd
Menorrx LLC
Merck & Co Inc
Merck Serono SA
NextGem Inc
Nipro Corp
Novomedics L L C
OCON Medical Ltd
OncoGenesis
Otago Innovation Ltd
Pfizer Inc
Sebela Pharmaceuticals Inc
Shandong Junxiu Biotechnology Co Ltd
Teva Pharmaceuticals USA Inc
Teva Women's Health
University of California San Diego
University of Cape Town
University of Minnesota
University of Oxford
University of Texas at Austin
University of Texas Health Science Center at San Antonio
University of Washington
Womed
Yale University
ZR-Operculum, Inc.
Table of Contents
Table
Figures
Frequently asked questions
-
Which was the leading segment in the reproductive health devices pipeline market as of February?
Orthopedic braces and supports was the leading segment in the reproductive health devices pipeline market as of February 2024.
-
Which territory had the highest number of products in the reproductive health devices pipeline market as of February 2024?
As of February 2024, the US accounted for the highest number of products in the reproductive health devices pipeline market.
-
Which was the most followed regulatory pathway in the reproductive health devices pipeline market as of February 2024?
As of February 2024, 510(k) was the most followed pathway for pipeline products in the reproductive health devices market.
-
Which are the major companies operating in the reproductive health devices pipeline market?
A few of the key companies associated with the reproductive health devices pipeline market are AdneXXA LLC, Agile Therapeutics Inc, AltaScience Limited, Bayer AG, Bioceptive Inc, and Biorings LLC, among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Reproductive Health Devices reports