Respiratory Syncytial Virus Prophylaxis – Global Drug Forecast and Market Analysis to 2030
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Respiratory syncytial virus (RSV) is a common respiratory infection that most children will be infected with by the time they are two years old. RSV infection generally causes mild flu-like symptoms, which can include a runny nose, lowered appetite, fever, coughing, and/or sneezing. Irritability and difficulty breathing may be the only symptoms shown by very young infants with RSV. RSV is a viral infection that is transmitted through contact with droplets in the air or on surfaces like doorknobs, tabletops, and cribs, and through direct contact with the virus. Although RSV currently has neither a known cure nor a vaccine, preventive measures can be taken to help mitigate the spread of the disease.
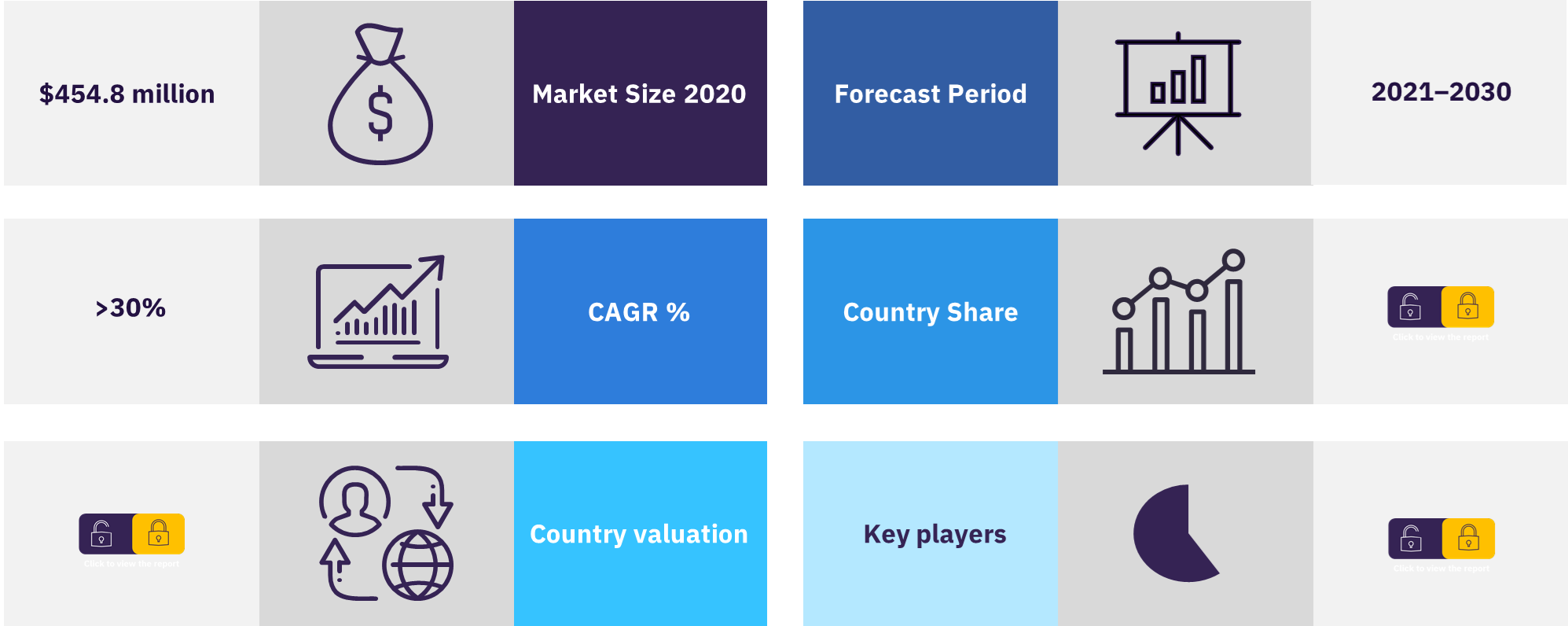
The RSV market is expected to experience significant growth over the forecast period (2020-2030) with the launches of numerous first-in-class products. The global prophylactic RSV market across the 8MM was valued at $454.8 million in 2020 and is expected to grow at an exponential CAGR of more than 30% over the 10-year forecast period. The RSV market’s growth is subjected to the launches of numerous first-in-class products.
The prophylactic market in the 8MM in 2020 consists of only one product-Sobi/AbbVie’s (formerly AstraZeneca’s) monoclonal antibody, Synagis (palivizumab). However, sales of Synagis are expected to drop substantially after the launch of competing passive prophylaxis products including AstraZeneca/Sanofi’s nirsevimab (MEDI-8897) and Merck’s clesrovimab (MK-1654).
Overview of global prophylactic RSV market
For more insights on this report, download a free report sample
What are the key countries in the global prophylactic RSV market?
The US was the leading country in the market followed by the UK, Germany, France, Japan, Italy, Australia, and Spain.
Global prophylactic RSV market, by countries
For more country insights on this report, download a free report sample
What are the market dynamics of global prophylactic RSV market?
The global prophylactic RSV market is driven by the projected launch of AstraZeneca/Sanofi’s nirsevimab across the 8MM, the first vaccines to reach the market to prevent medically significant RSV infections in infants and older adults, and the expectation that a range of prophylactic products will be needed to address the diversity of infection in different patient populations.
There is currently only one FDA-approved preventative product on the market-Sobi/AbbVie’s monoclonal antibody, Synagis (palivizumab). Key opinion leaders (KOLs) have identified the dosing burden of five monthly injections during RSV season and the high cost of Synagis as major areas for improvement. These factors contribute to a limited recommendation issued by the American Academy of Pediatrics (AAP) that restricts its use to only the highest-risk premature infants, including those with conditions such as congenital heart disease (CHD) and chronic lung disease (CLD). The adult prophylaxis market currently has no products available and will also experience rapid growth with the launch of several vaccines for third-trimester pregnant women and adults 60 years of age and older. Due to the high level of competition in RSV prophylaxis, products will need to differentiate themselves using innovative therapeutic approaches.
What is the competitive landscape and opportunities of the global prophylactic RSV market?
The competitive landscape of RSV therapies is characterized by a high level of unmet needs and opportunities. Although nearly all individuals are infected by RSV by the end of their second year of life, infants who are premature, immunocompromised, or who have chronic lung or heart disease are more likely to develop severe lower respiratory tract infections (LRTIs) as a result of infection.
Despite a surge in R&D for RSV prophylactics and antivirals over the past decade, the field has produced significantly more disappointments than success stories. Synagis remains the only licensed intervention for the prevention of RSV in infants at high risk of severe infection, and there are no approved prophylactic agents for adults or otherwise healthy young children. The short half-life and high cost of Synagis continue to be a major barrier to its widespread clinical use despite its efficacy in high-risk infants. Synagis is not used in current practice as a therapeutic intervention for the treatment of existing RSV infections, which means that there are effectively no targeted treatment options for RSV in the 8MM. Ribavirin, although approved for the treatment of RSV infections in infants and adults, is rarely used as its clinical efficacy has not reliably been demonstrated.
Most individuals at risk for moderate or severe RSV infections do not currently have any prophylactic options available. Synagis is only recommended for certain high-risk pediatric patients due to its high cost. Because a significant number of infants hospitalized for RSV infections do not belong to any of the high-risk categories that are eligible for prophylaxis, they are therefore not targeted for prophylaxis during the RSV season.
Who are the major players in the global prophylactic RSV market?
In the global prophylactic RSV market, the major players include AstraZeneca, Sobi Inc., AbbVie Inc., and AIMM Therapeutics.
Market report scope
| Market size (Year – 2020) | $454.8 million |
| Growth rate | CAGR of >30% from 2020 to 2030 |
| Base year for estimation | 2020 |
| Forecast period | 2020-2030 |
| Key Countries | The US, France, Spain, Germany, Italy, United Kingdom, Japan and Australia |
| Key players | AstraZeneca, Sobi Inc., AbbVie Inc., AIMM Therapeutics, GlaxoSmithKline, Pfizer, Johnson & Johnson, Sanofi, Merck, and Bavarian Nordic |
This report provides a comprehensive analysis of the global prophylactic RSV market. It provides:
- Overview of RSV, including epidemiology, etiology, pathophysiology, symptoms, diagnosis, and current management strategies.
- Topline RSV prophylaxis market revenue from 2020-2030. The annual cost of therapy and major pipeline product sales in this forecast period are included.
- Key topics covered include current RSV prophylaxis, unmet needs and opportunities, and the drivers and barriers affecting CHB treatment sales in the 8MM.
- Pipeline analysis: comprehensive data split across different phases, emerging novel trends under development, synopses of innovative early-stage projects, and detailed analysis of late-stage pipeline products.
- Analysis of the current and future market competition in the global RSV prophylaxis market. Insightful review of the key industry drivers, constraints, and challenges. Each trend is independently researched to provide a qualitative analysis of its implications.
Reasons to Buy
The report will enable you to:
- Develop and design your in-licensing and out-licensing strategies through a review of pipeline products and technologies, and by identifying the companies with the most robust pipeline.
- Develop business strategies by understanding the trends shaping and driving the global RSV prophylactic market.
- Drive revenues by understanding the key trends, innovative products and technologies, market segments, and companies likely to impact the RSV prophylaxis market in the future.
- Formulate effective sales and marketing strategies by understanding the competitive landscape and by analyzing the performance of various competitors.
- Identify emerging players with potentially strong product portfolios and create effective counterstrategies to gain a competitive advantage.
- Organize your sales and marketing efforts by identifying the market categories and segments that present maximum opportunities for consolidations, investments, and strategic partnerships.
Abbvie Inc.
Sobi Inc.
Sanofi
Merck & Co
Pfizer Inc.
GlaxoSmithKline
Johnson & Johnson
Bavarian Nordic
Table of Contents
Table
Figures
Frequently asked questions
-
What was the global prophylactic RSV market value in 2020?
The total global prophylactic RSV market value was $454.8 million in 2020.
-
What is the growth rate of the global prophylactic RSV market?
The global prophylactic RSV market is projected to grow at a CAGR of more than 30% during the period 2020-2030.
-
What are the key countries in the global prophylactic RSV market?
The US was the leading country in the market followed by the UK, Germany, France, Japan, Italy, Australia, and Spain.
-
Who are the key players in the global prophylactic RSV market?
The current major players in the market include AstraZeneca, Sobi Inc., AbbVie Inc. and AIMM Therapeutics.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Infectious Disease reports