Surgical Adhesion Barriers Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Surgical Adhesion Barriers Pipeline Market Report Overview
Adhesions are bands of scar-like tissue that forms abnormal fibrous connections joining tissue surfaces in abnormal locations. In general, any type of transperitoneal operation can lead to the formation of adhesions ranging from minimal scarring of serosal surface to firm agglutination of nearly all structures. However, post-surgical adhesion has a considerable epidemiological and clinical impact mostly due to tissue damage caused by surgical traumas.
The surgical adhesion barriers pipeline market research report provides a comprehensive understanding of the pipeline products along with their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Segments | · Gel Surgical Adhesion Barriers
· Membrane Surgical Adhesion Barriers |
| Key Territories | · The US
· Europe · Japan · Singapore · Taiwan |
| Key Regulatory Paths | · 510(k)
· CE · HSA · BOPA · NIFDS |
| Leading Companies | · Actamax Surgical Materials LLC
· Alafair Biosciences Inc · Anika Therapeutics Inc · Dalim Tissen Co Ltd · Duke University |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Surgical Adhesion Barriers Pipeline Market by Segments
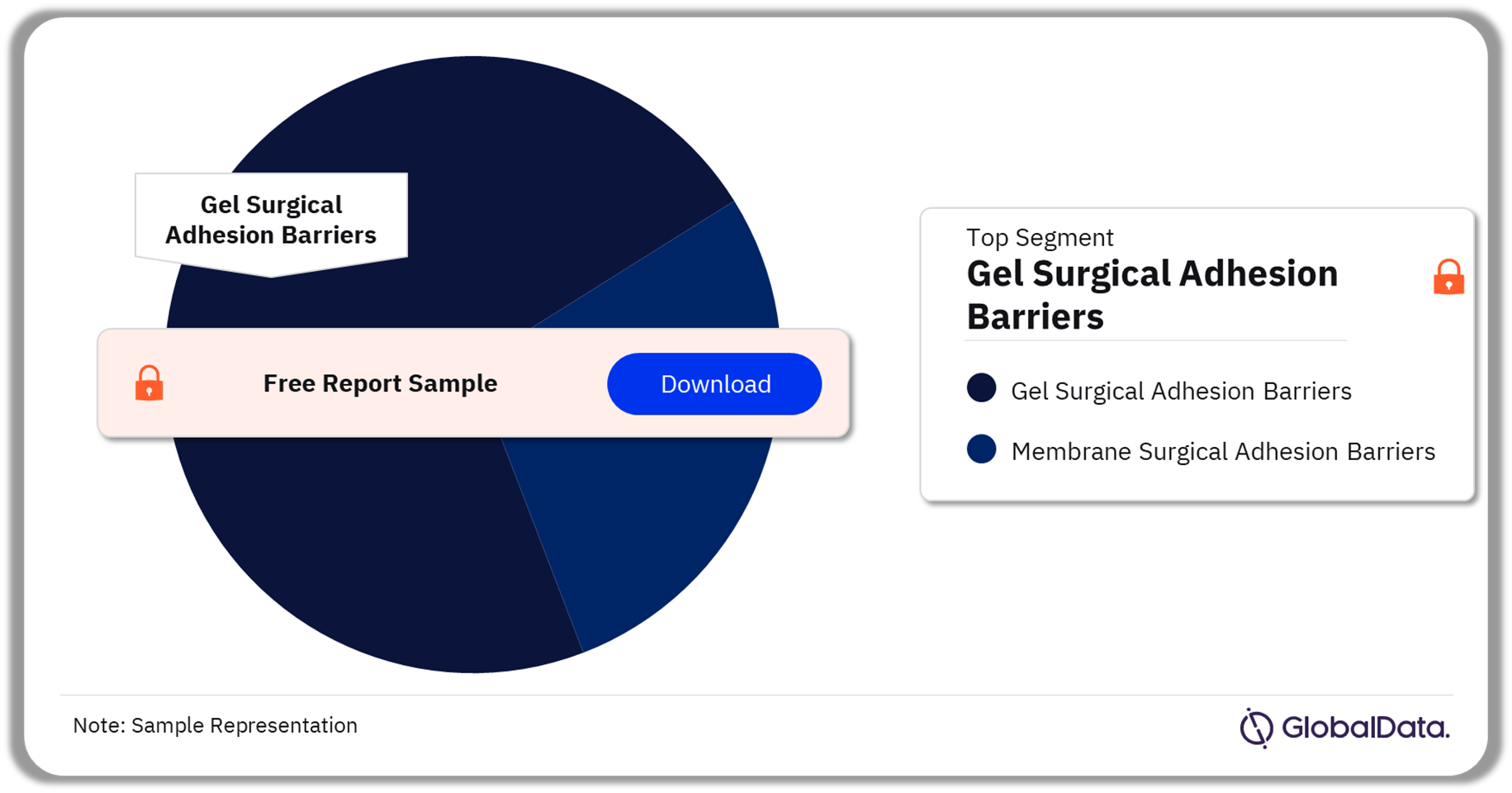
The key segments in the surgical adhesion barriers pipeline market are gel surgical adhesion barriers and membrane surgical adhesion barriers. As of December 2023, gel surgical adhesion barriers were the leading segment accounting for the highest number of products in the pipeline.
Surgical Adhesion Barriers Pipeline Market Analysis by Segments, 2023 (%)
Buy the Full Report for More Segment Insights into the Surgical Adhesion Barriers Pipeline Market
Surgical Adhesion Barriers Pipeline Market Segmentation by Territories
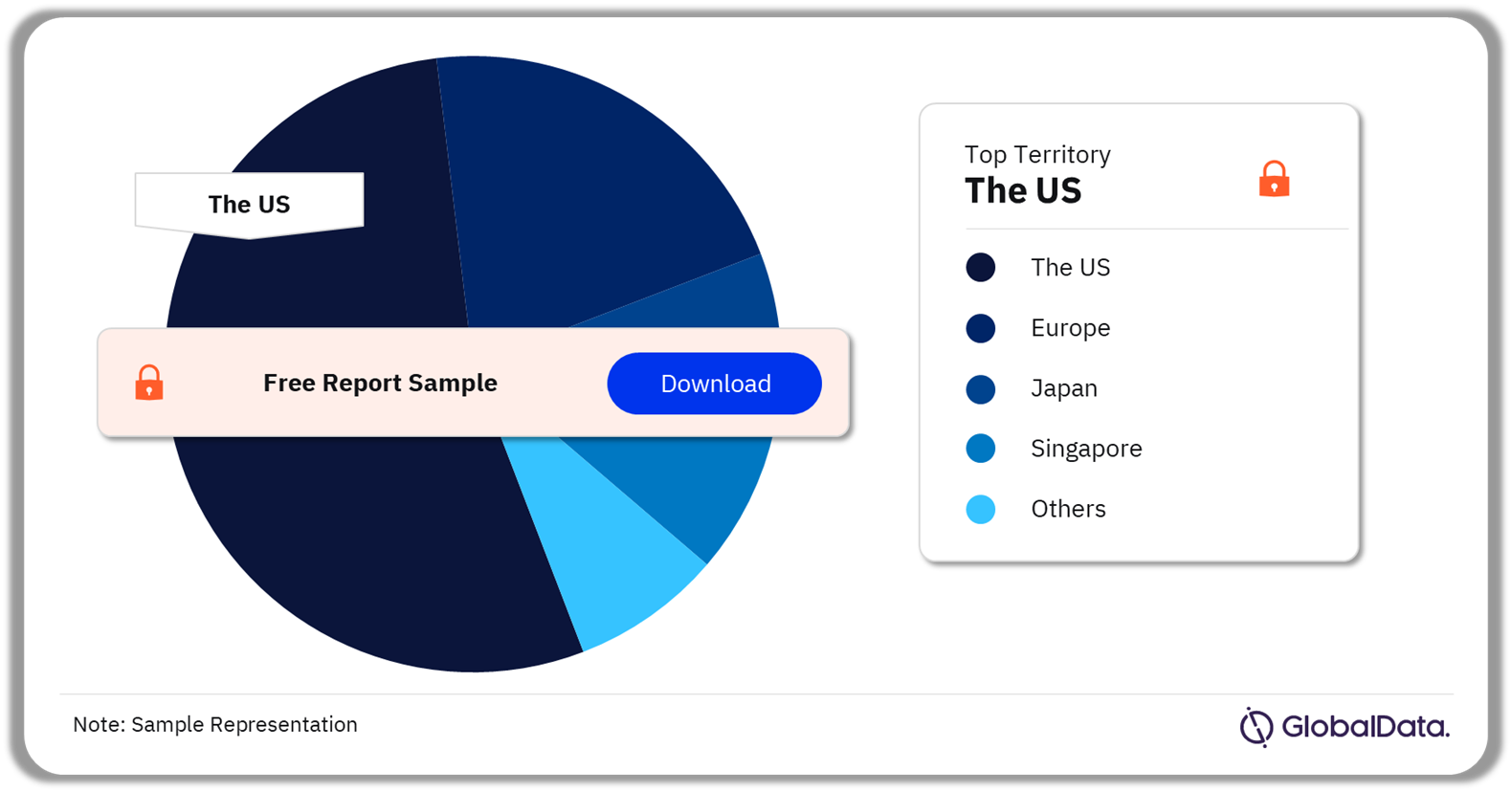
A few of the key territories for surgical adhesion barriers pipeline products are the US, Europe, Japan, Singapore, and Taiwan among others. As of December 2023, the US accounted for the highest number of pipeline products.
Surgical Adhesion Barriers Pipeline Market Analysis by Territories, 2023 (%)
Buy the Full Report for More Territory Insights into the Surgical Adhesion Barriers Pipeline Market
Surgical Adhesion Barriers Pipeline Market Segmentation by Regulatory Paths
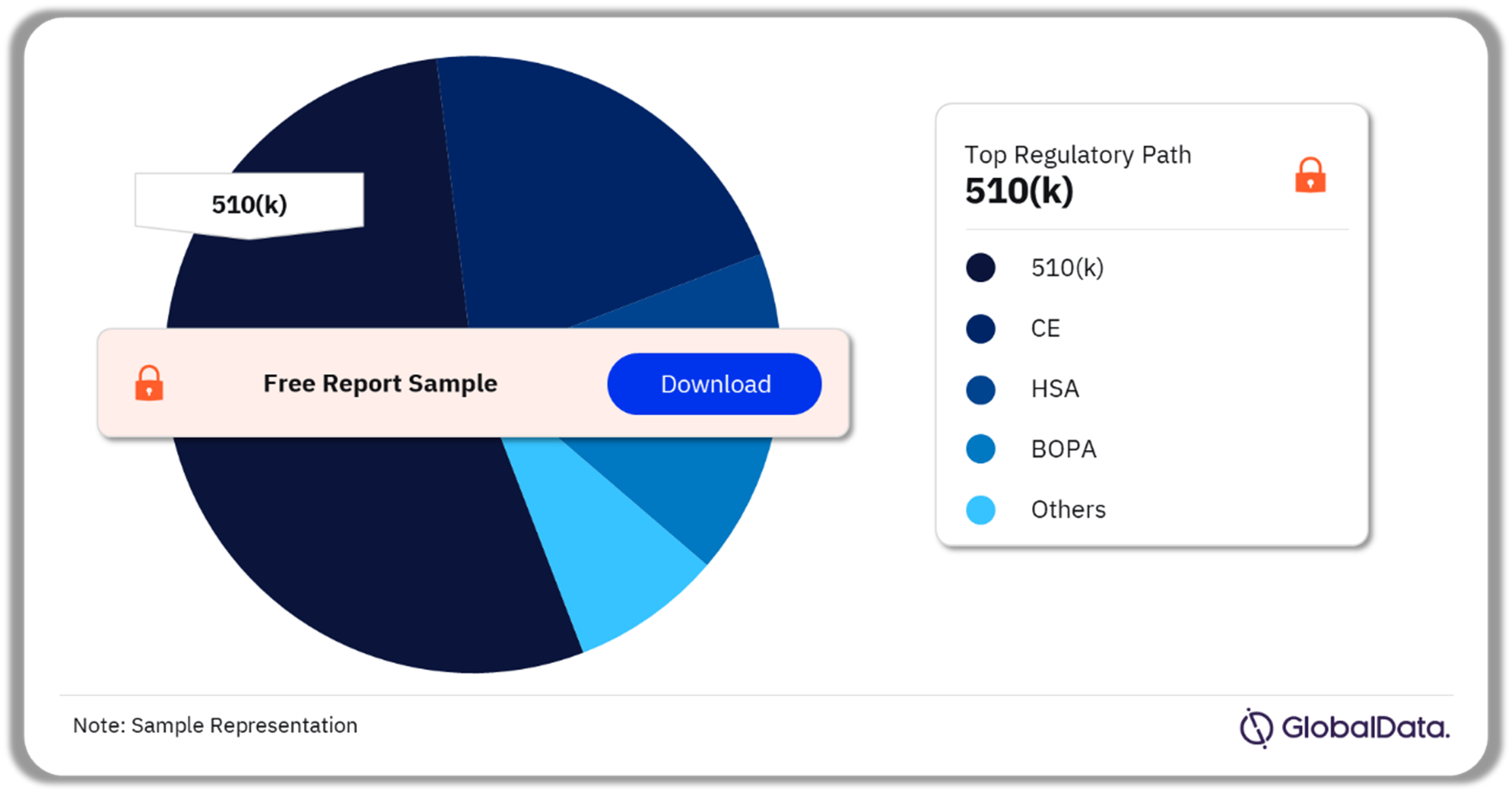
A few of the key regulatory paths followed by the surgical adhesion barriers pipeline market are 510(k), CE, HSA, BOPA, NIFDS. As of December 2023, 510(k) was the most followed pathway for pipeline products in the market.
Surgical Adhesion Barriers Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy the Full Report for More Regulatory Path Insights into the Surgical Adhesion Barriers Pipeline Market
Surgical Adhesion Barriers Pipeline Market – Competitive Landscape
A few of the key companies associated with the surgical adhesion barriers pipeline market are Actamax Surgical Materials LLC, Alafair Biosciences Inc, Anika Therapeutics Inc., Dalim Tissen Co Ltd, and Duke University among others.
Alafair Biosciences Inc: Alafair Biosciences is headquartered in Austin, Texas, the US. The company is a medical device company that develops and commercialize surgical products for soft tissue repair and protection applications. The company develops products based on a non-cell adhesive hydrogel technology to protect and manage advanced wound healing. It offers VersaWrap tendon protector, an ultrathin, sutureless, bioresorbable hydrogel that protects tendon gliding by separating injured tendon from surrounding tissues.
Anika Therapeutics Inc: Anika is headquartered in Bedford, Massachusetts, the US. The company develops, manufactures, and markets therapeutic products for degenerative orthopedic diseases and traumatic conditions. It offers solutions for ortho-biologics, dermal, ophthalmic, surgical, and veterinary applications. Its major products include human and veterinary visco-supplementation, Joint implants, instrumentation devices, joint replacement solutions, regenerative and orthopedic surgical therapies, bone repair therapy, advanced wound care, and ophthalmic products.
Surgical Adhesion Barriers Pipeline Market Analysis by Companies, 2023
Buy the Full Report for More Company Insights into the Surgical Adhesion Barriers Pipeline Market
Download a Free Report Sample
Segments Covered in the Report
Surgical Adhesion Barriers Pipeline Market Segments Outlook
- Gel Surgical Adhesion Barriers
- Membrane Surgical Adhesion Barriers
Surgical Adhesion Barriers Pipeline Market Territories Outlook
- The US
- Europe
- Japan
- Singapore
- Taiwan
Surgical Adhesion Barriers Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE
- HSA
- BOPA
- NIFDS
Scope
This report provides:
- Extensive coverage of the surgical adhesion barriers under development.
- Exhaustive list of major pipeline products and their details, including product description, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of surgical adhesion barriers and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of surgical adhesion barriers under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out in-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Alafair Biosciences Inc
Anika Therapeutics Inc
ARC Medical Devices Inc
Baxter International Inc
BioActive Polymers in Lund AB
Dalim Tissen Co Ltd
Duke University
FzioMed Inc
Genzyme Corp
HydroGlaze BV
Innocoll Technologies Ltd (Inactive)
Jinwoo Bio Co Ltd
Kytogenics Pharmaceuticals Inc
Leader Biomedical Europe BV
Luna Innovations Inc
Magen OrthoMed Ltd
Maxigen Biotech Inc
Medisse BV (Inactive)
MLM Biologics Inc
PolyNovo Biomaterials Pty Ltd
Rutgers The State University of New Jersey
Seikagaku Corp
Sporogenics Pte Ltd
Stony Brook University
Toray Medical Co Ltd
Tribos LLC
TYBR Health Inc
University of North Carolina at Chapel Hill
Table of Contents
Table
Figures
Frequently asked questions
-
Which was the leading segment in the surgical adhesion barriers pipeline market in 2023?
Gel surgical adhesion barriers was the leading segment in the surgical adhesion barriers pipeline market as of December 2023.
-
Which territory had the highest number of products in the surgical adhesion barriers pipeline market in 2023?
As of December 2023, the US accounted for the highest number of products in the surgical adhesion barriers pipeline market.
-
Which was the most followed regulatory pathway in the surgical adhesion barriers pipeline market in 2023?
As of December 2023, 510(k) was the most followed pathway for pipeline products in the surgical adhesion barriers market.
-
Which are the major companies operating in the surgical adhesion barriers pipeline market?
A few of the key companies associated with the surgical adhesion barriers pipeline market are Actamax Surgical Materials LLC, Alafair Biosciences Inc, Anika Therapeutics Inc., Dalim Tissen Co Ltd, and Duke University.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Surgical Adhesion Barriers reports