Surgical Devices – Pipeline Products by Stage of Development 29
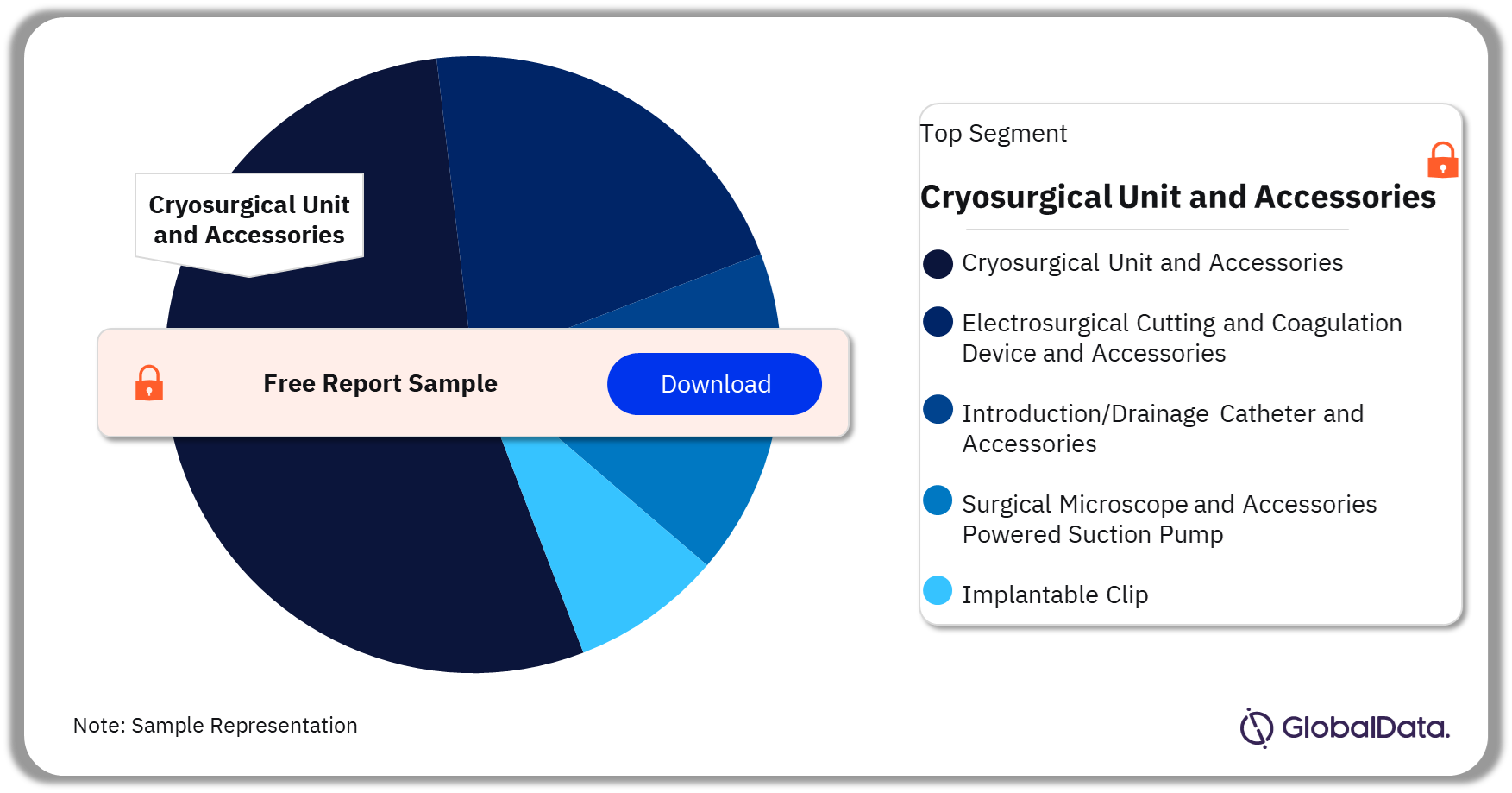
Surgical Devices – Pipeline Products by Segment 30
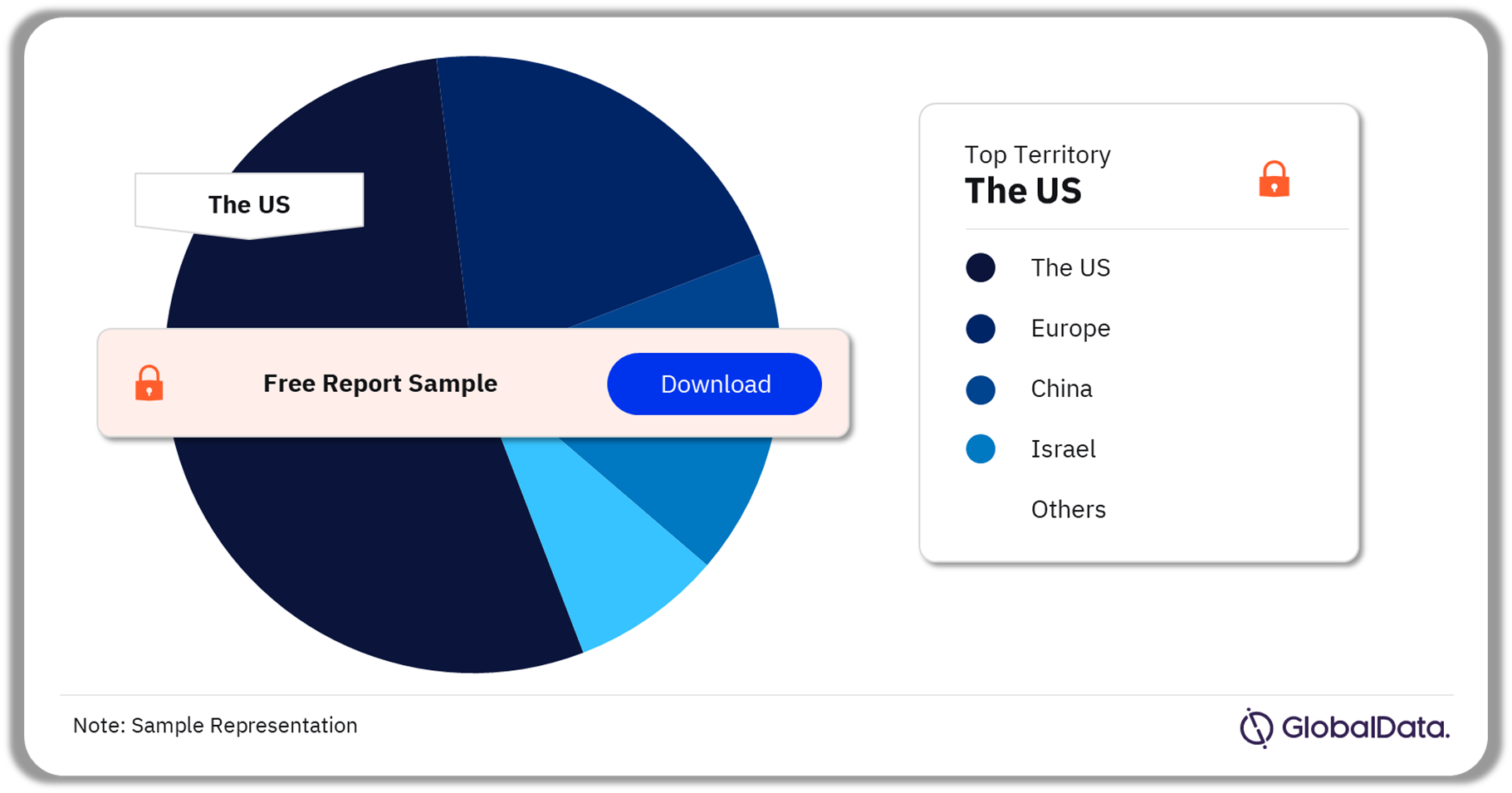
Surgical Devices – Pipeline Products by Territory 31
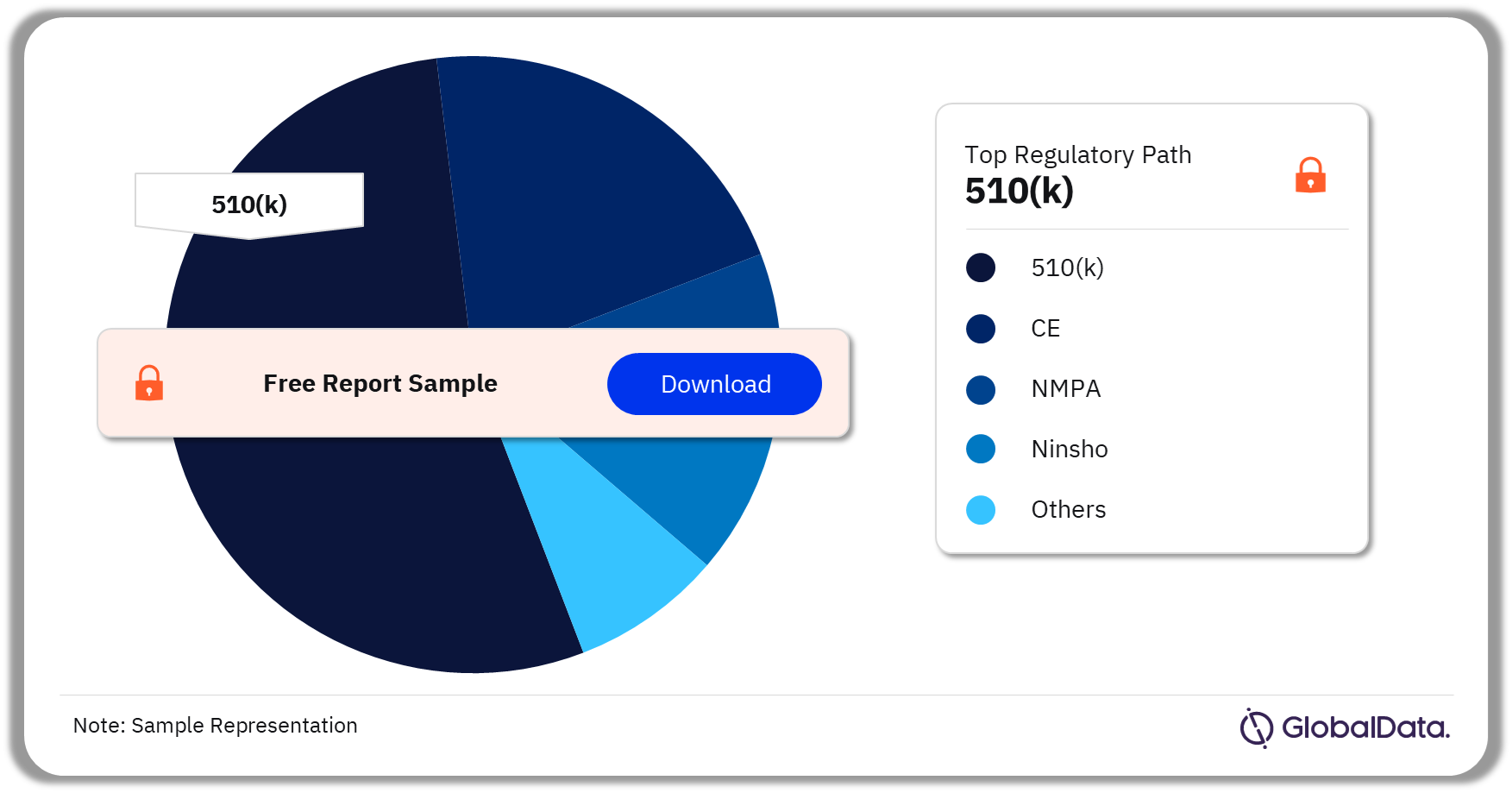
Surgical Devices – Pipeline Products by Regulatory Path 32
Surgical Devices – Pipeline Products by Estimated Approval Date 33
Surgical Devices – Ongoing Clinical Trials 34
Surgical Devices Companies – Pipeline Products by Stage of Development 35
Surgical Devices – Pipeline Products by Stage of Development 42
6S Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 48
TroClosure – Product Status 48
TroClosure – Product Description 48
Abbott Medical Pipeline Products & Ongoing Clinical Trials Overview 49
Ablation Catheter – Product Status 49
Ablation Catheter – Product Description 49
Abbott Operations Uruguay Srl Pipeline Products & Ongoing Clinical Trials Overview 50
Formosseo – Product Status 50
Formosseo – Product Description 50
AbSolutions Med Inc Pipeline Products & Ongoing Clinical Trials Overview 51
REBUILD System – Product Status 51
REBUILD System – Product Description 51
AbSolutions Med Inc – Ongoing Clinical Trials Overview 52
REBUILD System – The REBUILD Trial: A Prospective, Multi-Center, Single Arm Study Using REBUILD for Abdominal Wall Closure 53
Acoustic MedSystems Inc Pipeline Products & Ongoing Clinical Trials Overview 54
ACOUSTx-Flexible Catheter – Benign Prostatic Hyperplasia – Product Status 54
ACOUSTx-Flexible Catheter – Benign Prostatic Hyperplasia – Product Description 54
ACOUSTx-Flexible Catheter – Lung Cancer – Product Status 55
ACOUSTx-Flexible Catheter – Lung Cancer – Product Description 55
ACOUSTx-Needle Catheter – Brain Tumor – Product Status 55
ACOUSTx-Needle Catheter – Brain Tumor – Product Description 56
ACOUSTx-Needle Catheter – Liver Cancer – Product Status 56
ACOUSTx-Needle Catheter – Liver Cancer – Product Description 56
Aesculap Inc Pipeline Products & Ongoing Clinical Trials Overview 57
MicroCutter XCHANGE 30 Generation 4 Device – Product Status 57
MicroCutter XCHANGE 30 Generation 4 Device – Product Description 57
Anastom Surgical Pipeline Products & Ongoing Clinical Trials Overview 58
UroLink – Product Status 58
UroLink – Product Description 58
Anchora Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 59
AnchoFix – Product Status 59
AnchoFix – Product Description 59
Apercure Surgical Ltd Pipeline Products & Ongoing Clinical Trials Overview 60
Surgical Drain Retention Device – Product Status 60
Surgical Drain Retention Device – Product Description 60
Apollo Endosurgery Inc Pipeline Products & Ongoing Clinical Trials Overview 61
Overstitch – Endoscopic Sleeve Gastroplasty – Product Status 61
Overstitch – Endoscopic Sleeve Gastroplasty – Product Description 61
Applied Medical Resources Corporation Pipeline Products & Ongoing Clinical Trials Overview 62
Next Generation Colorectal Surgical Device – Product Status 62
Next Generation Colorectal Surgical Device – Product Description 62
Next Generation Gynecologic Surgical Device – Product Status 63
Next Generation Gynecologic Surgical Device – Product Description 63
Next Generation Obstetric Surgical Device – Product Status 63
Next Generation Obstetric Surgical Device – Product Description 63
Next Generation Urologic Surgical Device – Product Status 64
Next Generation Urologic Surgical Device – Product Description 64
Next Generation Vascular Surgical Device – Product Status 64
Next Generation Vascular Surgical Device – Product Description 65
Apyx Medical Corp Pipeline Products & Ongoing Clinical Trials Overview 66
Renuvion/J-Plasma Precise Handpiece – Product Status 66
Renuvion/J-Plasma Precise Handpiece – Product Description 67
Seal-N-Cut Handle – Product Status 67
Seal-N-Cut Handle – Product Description 67
Apyx Medical Corp – Ongoing Clinical Trials Overview 68
Renuvion/J-Plasma Precise Handpiece – Evaluation of the Use of the Renuvion APR System in the Labia 69
Archon Medical Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview 70
FastStitch Device – Product Status 70
FastStitch Device – Product Description 70
Augusta University Pipeline Products & Ongoing Clinical Trials Overview 71
Tissue Cutter – Product Status 71
Tissue Cutter – Product Description 71
Baltimore VA Medical Center Pipeline Products & Ongoing Clinical Trials Overview 72
Surgical Drain Positioning Device – Product Status 72
Surgical Drain Positioning Device – Product Description 72
Ultraviolet Sterilizing Drainage Catheter – Product Status 73
Ultraviolet Sterilizing Drainage Catheter – Product Description 73
Baxter International Inc Pipeline Products & Ongoing Clinical Trials Overview 74
Gem zipclip – Product Status 74
Gem zipclip – Product Description 74
BioTex Inc Pipeline Products & Ongoing Clinical Trials Overview 75
Laser Balloon – Product Status 75
Laser Balloon – Product Description 75
BriteSeed Pipeline Products & Ongoing Clinical Trials Overview 76
SafeSnips – Product Status 76
SafeSnips – Product Description 76
BT BeaMedical Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 77
StingRay Laser System – Product Status 77
StingRay Laser System – Product Description 77
BTG International Inc Pipeline Products & Ongoing Clinical Trials Overview 78
Cryotherapy Ablation System – Bone and Soft Tissue – Product Status 78
Cryotherapy Ablation System – Bone and Soft Tissue – Product Description 78
Cryotherapy Ablation System – Liver Metastasis – Product Status 79
Cryotherapy Ablation System – Liver Metastasis – Product Description 79
Cryotherapy Ablation System – Lung Metastasis – Product Status 79
Cryotherapy Ablation System – Lung Metastasis – Product Description 80
Carnegie Mellon University Pipeline Products & Ongoing Clinical Trials Overview 81
Cryopreservation Visualization Device – Product Status 81
Cryopreservation Visualization Device – Product Description 81
Temperature Sensor – Thermal Surgery – Product Status 82
Temperature Sensor – Thermal Surgery – Product Description 82
Centre Hospitalier Universitaire de Nice Pipeline Products & Ongoing Clinical Trials Overview 83
Surgical Suture Gun – Product Status 83
Surgical Suture Gun – Product Description 83
Charite University Hospital of Berlin Pipeline Products & Ongoing Clinical Trials Overview 84
Non-Linear Stapler Device – Product Status 84
Non-Linear Stapler Device – Product Description 84
Ready-Prepared Surgical Knot Device – Product Status 85
Ready-Prepared Surgical Knot Device – Product Description 85
Chengdu Yurui Innovation Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 86
SurgSmart Intelligent Surgical Navigation System – Product Status 86
SurgSmart Intelligent Surgical Navigation System – Product Description 86
Colospan Ltd Pipeline Products & Ongoing Clinical Trials Overview 87
CG-100 – Product Status 87
CG-100 – Product Description 87
Control Medical Technology LLC Pipeline Products & Ongoing Clinical Trials Overview 88
Aspire Maspirator – Product Status 88
Aspire Maspirator – Product Description 88
Core Essence Orthopaedics, LLC Pipeline Products & Ongoing Clinical Trials Overview 89
nABLE Percutaneous Suture Management System – Product Status 89
nABLE Percutaneous Suture Management System – Product Description 89
Corporis Medical Pipeline Products & Ongoing Clinical Trials Overview 90
Mediclose System – Product Status 90
Mediclose System – Product Description 90
Covidien Ltd Pipeline Products & Ongoing Clinical Trials Overview 91
iNOLC Flexible Stapler – Product Status 91
iNOLC Flexible Stapler – Product Description 91
CR Bard Inc Pipeline Products & Ongoing Clinical Trials Overview 92
New Fixation Device – Product Status 92
New Fixation Device – Product Description 92
Transformational Fixation – Product Status 93
Transformational Fixation – Product Description 93
Creative Balloons GmbH Pipeline Products & Ongoing Clinical Trials Overview 94
hygh-tec access – Product Status 94
hygh-tec access – Product Description 94
Cryofocus Medtech Shanghai Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 95
Peri-Pulmonary Nodule Cryoablation System – Product Status 95
Peri-Pulmonary Nodule Cryoablation System – Product Description 95
CT Resources Inc Pipeline Products & Ongoing Clinical Trials Overview 96
Partial Cuff – Product Status 96
Partial Cuff – Product Description 96
Cypris Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 97
XACT Surgical Suture Device – Midface Descent – Product Status 97
XACT Surgical Suture Device – Midface Descent – Product Description 97
Dartmouth College Pipeline Products & Ongoing Clinical Trials Overview 98
Cubical Mechanical Device – Product Status 98
Cubical Mechanical Device – Product Description 98
Deep Blue Medical Advances Inc Pipeline Products & Ongoing Clinical Trials Overview 99
Anchor Clip – Product Status 99
Anchor Clip – Product Description 99
Drexel University Pipeline Products & Ongoing Clinical Trials Overview 100
Laparoscopic Tissue Cutter – Product Status 100
Laparoscopic Tissue Cutter – Product Description 100
Emblation Ltd Pipeline Products & Ongoing Clinical Trials Overview 101
Swift System – Product Status 101
Swift System – Product Description 101
Emblation Ltd – Ongoing Clinical Trials Overview 102
Swift System – Pilot Study to Determine the Safety and Efficacy of Regimen Frequencies Using the Swift Microwave Device for Mild to Moderate Toenail Onychomycosis Caused by Dermatophytes 103
Swift System – Pivotal Study to Assess the Clinical Efficacy and Safety of Microwave Treatment for Actinic Keratosis 103
Swift System – Pivotal Study to Assess the Clinical Efficacy and Safety of Microwave Treatment in Warts 103
Embricon Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 104
Varicose Vein Removal Device – Product Status 104
Varicose Vein Removal Device – Product Description 104
Endo Tools Therapeutics SA Pipeline Products & Ongoing Clinical Trials Overview 105
Soft Tissue Approximation Device – Product Status 105
Soft Tissue Approximation Device – Product Description 105
EndoEvolution, LLC Pipeline Products & Ongoing Clinical Trials Overview 106
EndoTransformer – Product Status 106
EndoTransformer – Product Description 106
Ethicon Endo-Surgery Inc Pipeline Products & Ongoing Clinical Trials Overview 107
ECHELON ONE Next Gen Shafts – Product Status 107
ECHELON ONE Next Gen Shafts – Product Description 107
Next Generation Open Linear Stapler – Product Status 108
Next Generation Open Linear Stapler – Product Description 108
ExpandoHeat LLC Pipeline Products & Ongoing Clinical Trials Overview 109
Forceps Device – Vessel Sealing – Product Status 109
Forceps Device – Vessel Sealing – Product Description 109
Fixit Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 110
Cingo Drainage Catheter Fixation Device – Product Status 110
Cingo Drainage Catheter Fixation Device – Product Description 110
Fogless International AB Pipeline Products & Ongoing Clinical Trials Overview 111
Laryngectomy Device – Product Status 111
Laryngectomy Device – Product Description 111
G.I. Windows Inc Pipeline Products & Ongoing Clinical Trials Overview 112
Incision-less Anastomosis System – Product Status 112
Incision-less Anastomosis System – Product Description 112
Magnetic Compression Anastomosis Device – Product Status 113
Magnetic Compression Anastomosis Device – Product Description 113
G.I. Windows Inc – Ongoing Clinical Trials Overview 114
Magnetic Compression Anastomosis Device – A Trial for a Procedure for Duodenal-Ileal Diversion with a Sleeve Gastrectomy for Patients With Obesity and Type 2 Diabetes Mellitus 115
Magnetic Compression Anastomosis Device – A Trial for a Procedure for Duodenum to Ileal Diversion to Treat Type 2 Diabetes 115
Magnetic Compression Anastomosis Device – Single Neodymium Magnet Anastomosis Procedure When Used to Create a Duodenal-Ileal Diversion for Subjects with Inadequate Weight Loss or Weight Regain Following Sleeve Gastrectomy 115
Galil Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 116
HEATfx Microwave Ablation System – Product Status 116
HEATfx Microwave Ablation System – Product Description 116
Garantis Pipeline Products & Ongoing Clinical Trials Overview 117
Surgical Instrument – Product Status 117
Surgical Instrument – Product Description 117
Genicon Inc Pipeline Products & Ongoing Clinical Trials Overview 118
Suture Loop Cannula – Product Status 118
Suture Loop Cannula – Product Description 118
Tissue Fusion Device – Product Status 119
Tissue Fusion Device – Product Description 119
Grand Valley State University Pipeline Products & Ongoing Clinical Trials Overview 120
Esophagus Dissection Device – Product Status 120
Esophagus Dissection Device – Product Description 120
H. Lee Moffitt Cancer Center & Research Institute Inc Pipeline Products & Ongoing Clinical Trials Overview 121
Muscle Stapler – Product Status 121
Muscle Stapler – Product Description 121
Pigtail Drainage Catheter – Product Status 122
Pigtail Drainage Catheter – Product Description 122
Hangzhou Jianjia Robot Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 123
Decaire Implant Navigation System – Product Status 123
Decaire Implant Navigation System – Product Description 123
Oral Implant Surgery Navigation and Positioning System – Product Status 124
Oral Implant Surgery Navigation and Positioning System – Product Description 124
Hangzhou Jianjia Robot Co Ltd – Ongoing Clinical Trials Overview 125
Decaire Implant Navigation System – Clinical Trial on the Safety and Effectiveness of the Navigation and Positioning System for Oral Implant Surgery in Clinical Application 126
Oral Implant Surgery Navigation and Positioning System – Clinical Trial on the Safety and Effectiveness of the Navigation and Positioning System for Oral Implant Surgery in Clinical Application 127
Oral Implant Surgery Navigation and Positioning System – Clinical Trial on the Safety and Effectiveness of the Navigation and Positioning System for Oral Implant Surgery in Clinical Application 127
Hangzhou Kangsheng Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 128
Automatic-loading Titanium Clips & Applier – Product Status 128
Automatic-loading Titanium Clips & Applier – Product Description 128
Hannover Medical School Pipeline Products & Ongoing Clinical Trials Overview 129
ACAD I – Product Status 129
ACAD I – Product Description 129
ACAD II – Product Status 130
ACAD II – Product Description 130
Pre-Sterilized Modular Surgical Guidance Device – Product Status 130
Pre-Sterilized Modular Surgical Guidance Device – Product Description 131
Harvard University Pipeline Products & Ongoing Clinical Trials Overview 132
Hybrid Snake Robot – Product Status 132
Hybrid Snake Robot – Product Description 132
Hospital for Special Surgery Pipeline Products & Ongoing Clinical Trials Overview 133
PRP Retention Implant – Product Status 133
PRP Retention Implant – Product Description 133
HydroCision Inc Pipeline Products & Ongoing Clinical Trials Overview 134
G.I.Jet – Product Status 134
G.I.Jet – Product Description 134
IceCure Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 135
IceSense3 Cryoprobe – Product Status 135
IceSense3 Cryoprobe – Product Description 136
lceSense3 – Product Status 136
lceSense3 – Product Description 136
Next Generation MultiSense System – Product Status 137
Next Generation MultiSense System – Product Description 137
Next Generation Single Probe System – Product Status 137
Next Generation Single Probe System – Product Description 138
ProSense System – Product Status 138
ProSense System – Product Description 138
IceCure Medical Ltd – Ongoing Clinical Trials Overview 139
lceSense3 – Cryoablation of Low Risk Breast Cancer Less than 1.5 cm: An Evaluation of Local Recurrence: Ice3 Trial 140
ProSense System – Cryoablation of Low Risk Breast Cancer Less than 1.5 cm: An Evaluation of Local Recurrence: Ice3 Trial 141
ProSense System – ICE-SECRET PROSENSE Cryotherapy for Renal Cell Carcinoma Trial 141
ProSense System – Safety and Effectiveness of Non Resection Ultrasound Guided Cryotherapy for Localized Early Breast Cancer 141
Imperial College London Pipeline Products & Ongoing Clinical Trials Overview 142
Surgical Clip And Applicator – Product Status 142
Surgical Clip And Applicator – Product Description 142
Indian Institute of Technology Delhi Pipeline Products & Ongoing Clinical Trials Overview 143
Surgical Stapler – Product Status 143
Surgical Stapler – Product Description 143
Innovex Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 144
High-Frequency Electrosurgical Instrument – Product Status 144
High-Frequency Electrosurgical Instrument – Product Description 144
Plasma Radio Frequency Generator (NW-100) – Product Status 145
Plasma Radio Frequency Generator (NW-100) – Product Description 145
Single-Use Electrosurgical Knife – Product Status 145
Single-Use Electrosurgical Knife – Product Description 146
Single-Use Electrosurgical Snare – Product Status 146
Single-Use Electrosurgical Snare – Product Description 146
Integra LifeSciences Holdings Corp Pipeline Products & Ongoing Clinical Trials Overview 147
CUSA Clarity 2 – Product Status 147
CUSA Clarity 2 – Product Description 147
Intuitive Surgical Inc Pipeline Products & Ongoing Clinical Trials Overview 148
Da Vinci Surgical Stapler Cartridge – Product Status 148
Da Vinci Surgical Stapler Cartridge – Product Description 148
IonMed Ltd. Pipeline Products & Ongoing Clinical Trials Overview 149
BioWeld1 System – Internal Tissue Fusion – Product Status 149
BioWeld1 System – Internal Tissue Fusion – Product Description 149
BioWeld1 System – Plastic Surgery – Product Status 150
BioWeld1 System – Plastic Surgery – Product Description 150
Jiangsu Bioperfectus Technologies Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 151
Kuicwell – Product Status 151
Kuicwell – Product Description 151
Jinan Micro Intelligent Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 152
Optical Device – Product Status 152
Optical Device – Product Description 152
Surgical Fluorescence Navigation System – Product Status 153
Surgical Fluorescence Navigation System – Product Description 153
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 154
Cartilage-Slicing Device – Product Status 154
Cartilage-Slicing Device – Product Description 154
Clamshell Wire Introducer – Product Status 155
Clamshell Wire Introducer – Product Description 155
Cutter System – Product Status 155
Cutter System – Product Description 155
GSump – Product Status 156
GSump – Product Description 156
Needle Drive Device – Product Status 156
Needle Drive Device – Product Description 157
Sump Facilitator – Product Status 157
Sump Facilitator – Product Description 157
Transfascial Suture Placement System – Product Status 158
Transfascial Suture Placement System – Product Description 158
joimax GmbH Pipeline Products & Ongoing Clinical Trials Overview 159
Vaporgrip Bipo – Product Status 159
Vaporgrip Bipo – Product Description 159
KARL STORZ SE & Co KG Pipeline Products & Ongoing Clinical Trials Overview 160
Next-Generation Instrumentation Device – Product Status 160
Next-Generation Instrumentation Device – Product Description 160
Keren Medical Ltd. Pipeline Products & Ongoing Clinical Trials Overview 161
AutoTy Anastomosis Device – Product Status 161
AutoTy Anastomosis Device – Product Description 161
AutoTy Closure Device – Product Status 162
AutoTy Closure Device – Product Description 162
AutoTy Fixation Device – Product Status 162
AutoTy Fixation Device – Product Description 163
Lausanne University Hospital Pipeline Products & Ongoing Clinical Trials Overview 164
Secure Knife – Product Status 164
Secure Knife – Product Description 164
LeMaitre Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 165
Elongated AnastoClip AC – Product Status 165
Elongated AnastoClip AC – Product Description 166
Elongated AnastoClip GC – Product Status 166
Elongated AnastoClip GC – Product Description 166
Livsmed Inc Pipeline Products & Ongoing Clinical Trials Overview 167
ArtiSential Bipolar Fenestrated Forceps – Product Status 167
ArtiSential Bipolar Fenestrated Forceps – Product Description 167
LuSeed Vascular Pipeline Products & Ongoing Clinical Trials Overview 168
LuSeed Intrasaccular Device – Liver Hemangioma – Product Status 168
LuSeed Intrasaccular Device – Liver Hemangioma – Product Description 168
Lymphatica Medtech SA Pipeline Products & Ongoing Clinical Trials Overview 169
LymphoPilot – Product Status 169
LymphoPilot – Product Description 169
Lymphatica Medtech SA – Ongoing Clinical Trials Overview 170
LymphoPilot – Pilot Clinical Study to Assess Safety and Feasibility of a New Implantable Device in the Management of Limb Lymphedema 171
MagniFeel Surgical Instruments Pipeline Products & Ongoing Clinical Trials Overview 172
MagniFeel Device – Product Status 172
MagniFeel Device – Product Description 172
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 173
Esophageal Endoscopic Mucosectomy Device – Product Status 173
Esophageal Endoscopic Mucosectomy Device – Product Description 173
Mecmaan Healthcare Ltd Pipeline Products & Ongoing Clinical Trials Overview 174
Parasafe – Product Status 174
Parasafe – Product Description 174
Medical University of South Carolina Pipeline Products & Ongoing Clinical Trials Overview 175
VATS Decortication Instrument – Product Status 175
VATS Decortication Instrument – Product Description 175
MediShield B.V. Pipeline Products & Ongoing Clinical Trials Overview 176
MSIS (MediShield Isolator System) Trocar System – Product Status 176
MSIS (MediShield Isolator System) Trocar System – Product Description 176
Medtronic Navigation Inc Pipeline Products & Ongoing Clinical Trials Overview 177
Stealthsphere – Product Status 177
Stealthsphere – Product Description 177
Mel Frontier Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 178
Colon Suture Staple – Product Status 178
Colon Suture Staple – Product Description 178
Meta Biomed Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 179
Biodegradable Anastomosis Device – Product Status 179
Biodegradable Anastomosis Device – Product Description 179
Biodegradable Surgical Clip – Product Status 180
Biodegradable Surgical Clip – Product Description 180
Biodegradable Suture Thread – Product Status 180
Biodegradable Suture Thread – Product Description 180
Non-Degradable Suture Thread – Product Status 181
Non-Degradable Suture Thread – Product Description 181
Michigan State University Pipeline Products & Ongoing Clinical Trials Overview 182
Ingress – Egress Device – Product Status 182
Ingress – Egress Device – Product Description 182
Modendo Inc Pipeline Products & Ongoing Clinical Trials Overview 183
Multi-Probe Ultrathin Endomicroscope – Product Status 183
Multi-Probe Ultrathin Endomicroscope – Product Description 183
Nanjing Premier Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 184
Absorbable Skull Locks – Product Status 184
Absorbable Skull Locks – Product Description 184
Herringbone Thread – Product Status 185
Herringbone Thread – Product Description 185
Naval Medical Research Center Pipeline Products & Ongoing Clinical Trials Overview 186
Antimicrobial Electrostatic Implantable Device – Product Status 186
Antimicrobial Electrostatic Implantable Device – Product Description 186
Endotracheal Tube Stabilization Device – Product Status 187
Endotracheal Tube Stabilization Device – Product Description 187
Next Science Ltd Pipeline Products & Ongoing Clinical Trials Overview 188
White Label Version – Xperience – Product Status 188
White Label Version – Xperience – Product Description 188
NorthShore University Health System Pipeline Products & Ongoing Clinical Trials Overview 189
Surgical Device – Product Status 189
Surgical Device – Product Description 189
NOvate Medical Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview 190
InfaClip – Product Status 190
InfaClip – Product Description 190
NoviRad Inc Pipeline Products & Ongoing Clinical Trials Overview 191
SmartDrain – Product Status 191
SmartDrain – Product Description 191
NovoGI LTD Pipeline Products & Ongoing Clinical Trials Overview 192
BowelRing – Product Status 192
BowelRing – Product Description 192
Novolock Ltd Pipeline Products & Ongoing Clinical Trials Overview 193
Fascia Closure Device – Product Status 193
Fascia Closure Device – Product Description 193
Novuson Surgical, Inc. Pipeline Products & Ongoing Clinical Trials Overview 194
10 mm UltraStat MAXX LS/OPEN – Product Status 194
10 mm UltraStat MAXX LS/OPEN – Product Description 194
3 mm UltraStat Mini LS – Product Status 195
3 mm UltraStat Mini LS – Product Description 195
Active Hemostatic Clamping Device – Product Status 195
Active Hemostatic Clamping Device – Product Description 196
NuEyes Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 197
NuLoupes – Product Status 197
NuLoupes – Product Description 197
Ohio State University Pipeline Products & Ongoing Clinical Trials Overview 198
Foldable Anvil Circular Surgical Stapler – Product Status 198
Foldable Anvil Circular Surgical Stapler – Product Description 198
Oklahoma State University Pipeline Products & Ongoing Clinical Trials Overview 199
Non-Invasive Device – Neurovascular Bundle – Product Status 199
Non-Invasive Device – Neurovascular Bundle – Product Description 199
Olympus America Inc Pipeline Products & Ongoing Clinical Trials Overview 200
Single-Use Nova Lighted Surgical Instrument – Product Status 200
Single-Use Nova Lighted Surgical Instrument – Product Description 200
OV World Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 201
Micro Suture – Product Status 201
Micro Suture – Product Description 201
Pacira BioSciences Inc Pipeline Products & Ongoing Clinical Trials Overview 202
Iovera – Lower Back Pain – Product Status 202
Iovera – Lower Back Pain – Product Description 203
Iovera – Rib Fracture – Product Status 203
Iovera – Rib Fracture – Product Description 203
Iovera – Spasticity – Product Status 204
Iovera – Spasticity – Product Description 204
Iovera – Specific Smart Tip – Product Status 204
Iovera – Specific Smart Tip – Product Description 204
Iovera – Spine Disease – Product Status 205
Iovera – Spine Disease – Product Description 205
Pacira BioSciences Inc – Ongoing Clinical Trials Overview 206
Iovera – Lower Back Pain – A Single-center, Randomized, Pilot Study to Assess Iovera° Lumbar Medial Branch Cryoneurolysis vs Lumbar Radiofrequency Ablation for Facet Mediated Chronic Low Back Pain 207
Parker Hannifin Corp Pipeline Products & Ongoing Clinical Trials Overview 208
The Navis Torquer – Product Status 208
The Navis Torquer – Product Description 208
Pavmed Inc Pipeline Products & Ongoing Clinical Trials Overview 209
EsoCure Esophageal Ablation Device – Product Status 209
EsoCure Esophageal Ablation Device – Product Description 209
PetVivo Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 210
Limb Salvage Shunt – Product Status 210
Limb Salvage Shunt – Product Description 210
Physcient, Inc. Pipeline Products & Ongoing Clinical Trials Overview 211
Differential Dissector, Model DDL – Product Status 211
Differential Dissector, Model DDL – Product Description 211
Model DD Micro Differential Dissector – Microsurgery – Product Status 212
Model DD Micro Differential Dissector – Microsurgery – Product Description 212
Plio Surgical Pipeline Products & Ongoing Clinical Trials Overview 213
Plio Surgical Implant – Product Status 213
Plio Surgical Implant – Product Description 213
PneumoNIX Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 214
CT-Guided Lung Biopsy Device – Product Status 214
CT-Guided Lung Biopsy Device – Product Description 214
Polytechnic University of Catalonia Pipeline Products & Ongoing Clinical Trials Overview 215
Anastomosis Device – Product Status 215
Anastomosis Device – Product Description 215
Pro-Dex Inc Pipeline Products & Ongoing Clinical Trials Overview 216
Staple Delivery Device – Product Status 216
Staple Delivery Device – Product Description 216
Queen Mary University of London Pipeline Products & Ongoing Clinical Trials Overview 217
Disposable Fistula Probe with Seton – Product Status 217
Disposable Fistula Probe with Seton – Product Description 217
Ramona Optics Inc Pipeline Products & Ongoing Clinical Trials Overview 218
M-CARE – Product Status 218
M-CARE – Product Description 218
Ratner BioMedical Inc Pipeline Products & Ongoing Clinical Trials Overview 219
BioCuff Sutureless Connector – Product Status 219
BioCuff Sutureless Connector – Product Description 219
Resultados y Calidad del Sistema Sanitario Publico de Andalucia Pipeline Products & Ongoing Clinical Trials Overview 220
Anastomosis Device – Product Status 220
Anastomosis Device – Product Description 220
Fast-Acting Emergency Tourniquet – Product Status 221
Fast-Acting Emergency Tourniquet – Product Description 221
Rice University Pipeline Products & Ongoing Clinical Trials Overview 222
TinyStitch Device – Product Status 222
TinyStitch Device – Product Description 222
RMIT University Pipeline Products & Ongoing Clinical Trials Overview 223
Smart Surgical Suture – Product Status 223
Smart Surgical Suture – Product Description 223
Saint Louis University Pipeline Products & Ongoing Clinical Trials Overview 224
Surgical Distractor – Product Status 224
Surgical Distractor – Product Description 224
SamanTree Technologies Pipeline Products & Ongoing Clinical Trials Overview 225
Histolog Scanner – Upgraded Version – Product Status 225
Histolog Scanner – Upgraded Version – Product Description 225
SamanTree Technologies – Ongoing Clinical Trials Overview 226
Histolog Scanner – Upgraded Version – Do the Results of Intra-operative Margin Assessment Correlate with the Output of Conventional Histology for Patient Undergoing Breast Conservation Surgery 227
Histolog Scanner – Upgraded Version – Intraoperative Assessment of Surgical Margins Using Confocal Microscopy in Comparison With Reference Extemporaneous Examination (Pilot Study) 227
Histolog Scanner – Upgraded Version – Reoperation Rate in Breast-Conserving Surgery Using Confocal Histolog Scanner for Intraoperative Margin Assessment in a Single-centric PMPF Study (SHIELD) 227
Samyang Biopharmaceuticals Corp Pipeline Products & Ongoing Clinical Trials Overview 228
Ligature Clip – Product Status 228
Ligature Clip – Product Description 228
Sanovas Inc Pipeline Products & Ongoing Clinical Trials Overview 229
CryoZeppelin – Product Status 229
CryoZeppelin – Product Description 229
ThermaZeppelin – Product Status 230
ThermaZeppelin – Product Description 230
Shanghai MicroPort Medical Group Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 231
Madam Curie – Product Status 231
Madam Curie – Product Description 231
Shanghai Ruidao Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 232
Irreversible Electroporation Ablation Device – Benign Prostatic Hyperplasia – Product Status 232
Irreversible Electroporation Ablation Device – Benign Prostatic Hyperplasia – Product Description 232
Irreversible Electroporation Ablation Device – Liver Tumor – Product Status 233
Irreversible Electroporation Ablation Device – Liver Tumor – Product Description 233
Shanghai Shengzhe Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 234
Sanpulse Radio Frequency UHF Surgery Integrated System – Product Status 234
Sanpulse Radio Frequency UHF Surgery Integrated System – Product Description 234
SapPare Endoscopic Electric Cutting and Closing Device – Product Status 235
SapPare Endoscopic Electric Cutting and Closing Device – Product Description 235
Shanghai Yichao Medical Equipment Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 236
Multimodal Mixed Energy Surgical System – Product Status 236
Multimodal Mixed Energy Surgical System – Product Description 236
Silmag SA Pipeline Products & Ongoing Clinical Trials Overview 237
Drainage Tube – Product Status 237
Drainage Tube – Product Description 237
Drainage Tube – Multi Lumen – Product Status 238
Drainage Tube – Multi Lumen – Product Description 238
simedeq Pipeline Products & Ongoing Clinical Trials Overview 239
Phlebectomy Device – Product Status 239
Phlebectomy Device – Product Description 239
Simon Fraser University Pipeline Products & Ongoing Clinical Trials Overview 240
Surgical Suturing Device – Product Status 240
Surgical Suturing Device – Product Description 240
StatLink Surgical Pipeline Products & Ongoing Clinical Trials Overview 241
Knotless Fastener – Product Status 241
Knotless Fastener – Product Description 241
Glossary
![]()