Therapeutic Drug Monitoring – Pipeline Products by Stage of Development 18
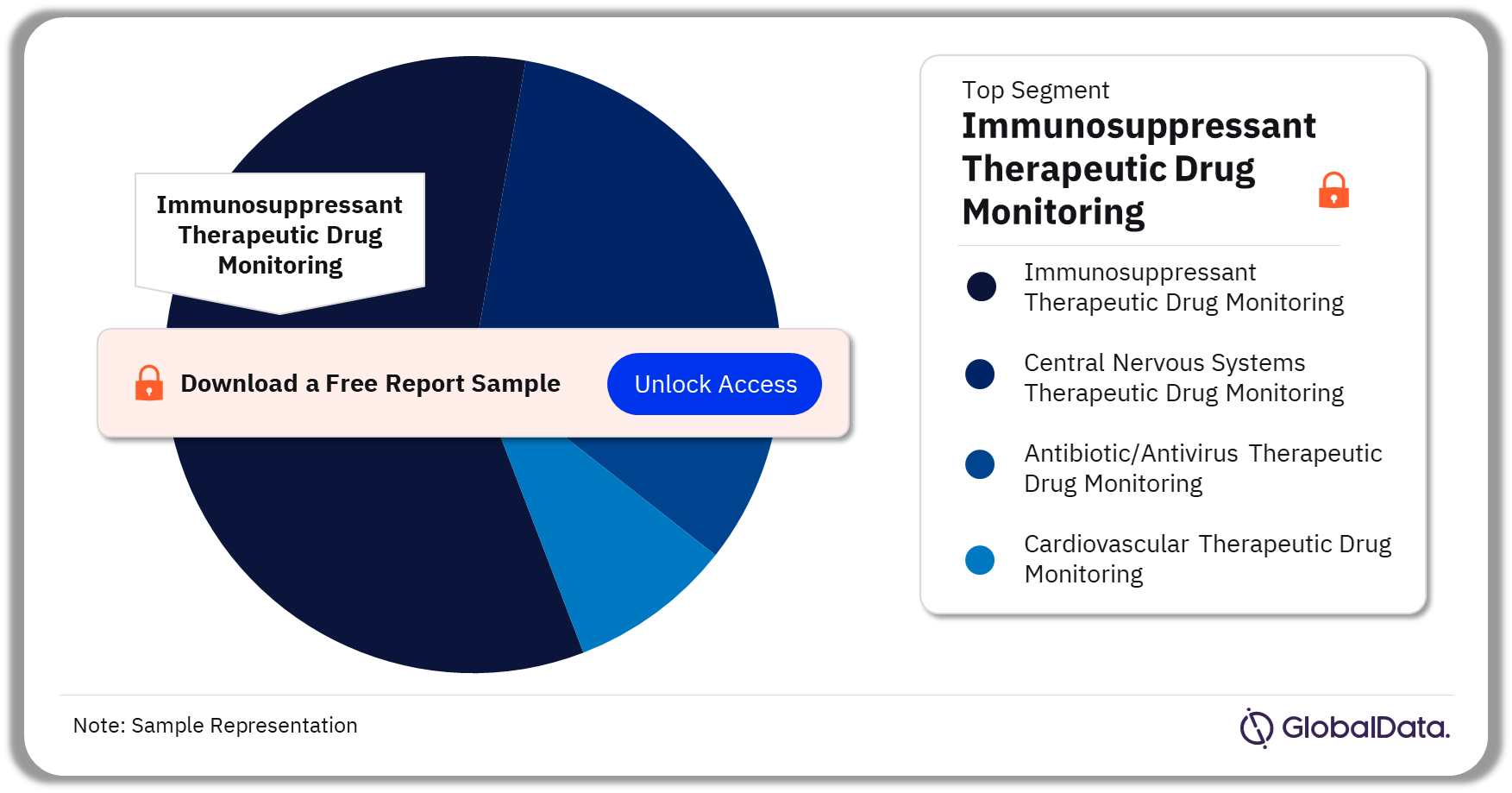
Therapeutic Drug Monitoring – Pipeline Products by Segment 19
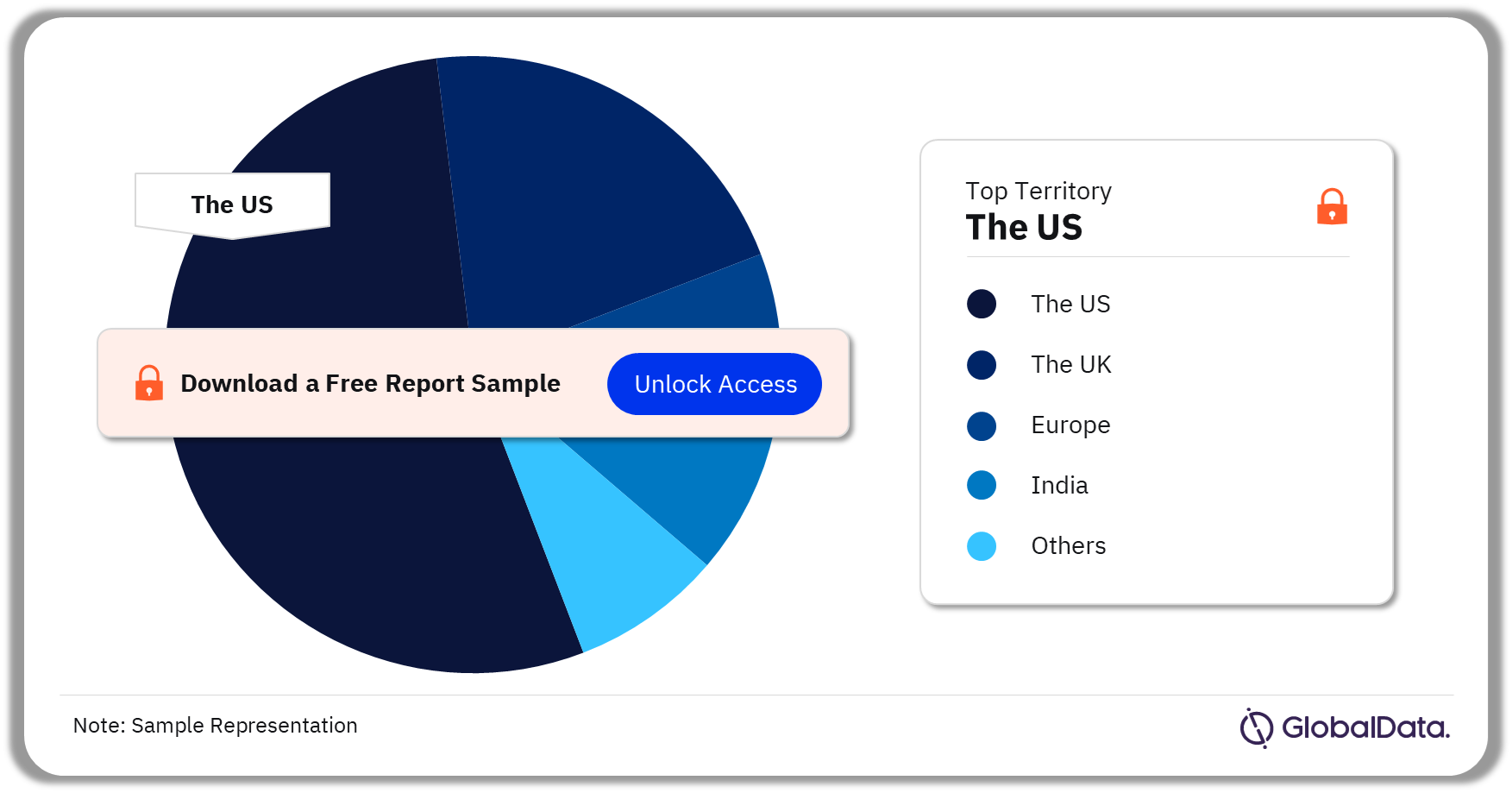
Therapeutic Drug Monitoring – Pipeline Products by Territory 20
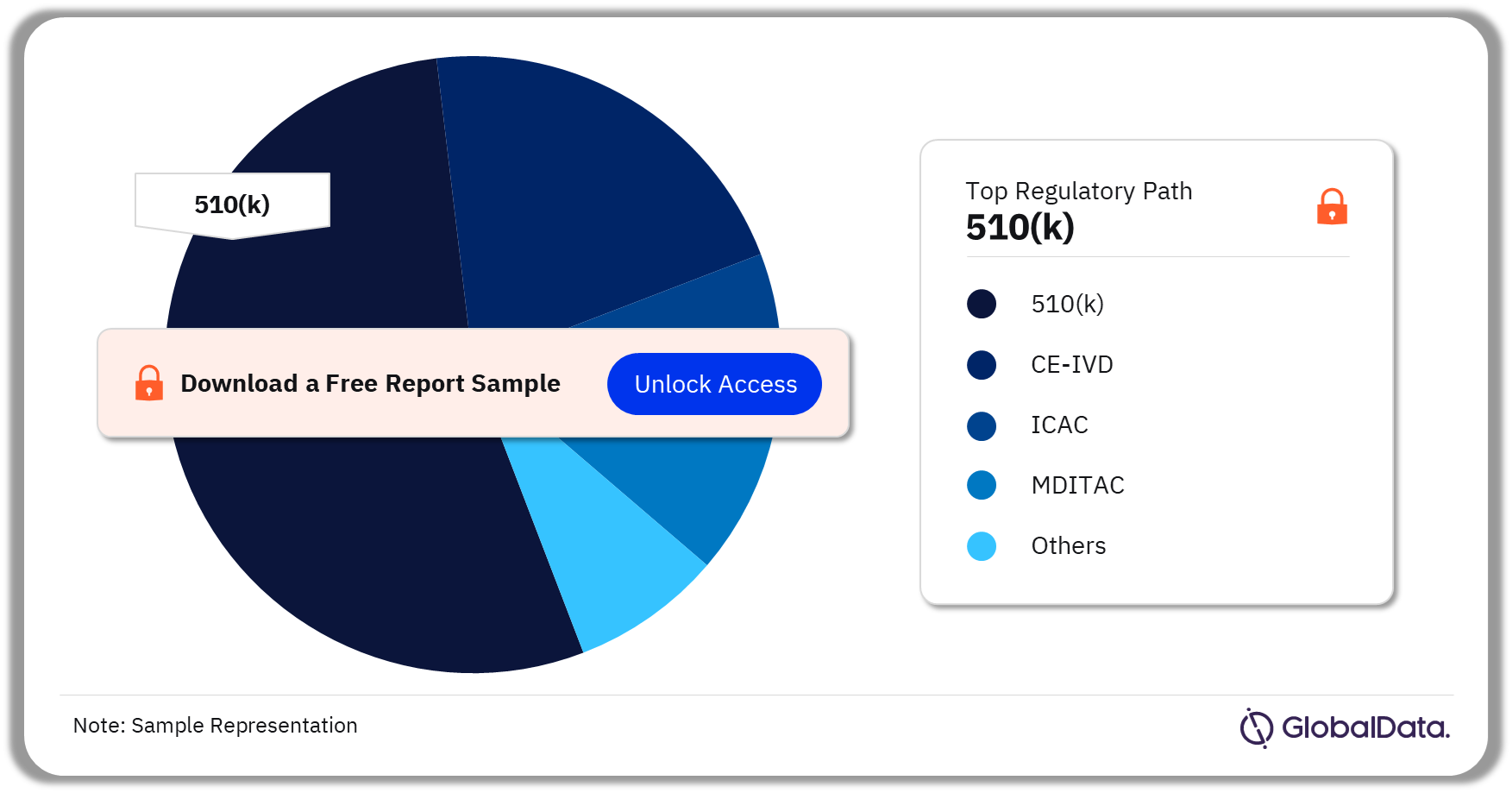
Therapeutic Drug Monitoring – Pipeline Products by Regulatory Path 21
Therapeutic Drug Monitoring – Pipeline Products by Estimated Approval Date 22
Therapeutic Drug Monitoring – Ongoing Clinical Trials 23
Therapeutic Drug Monitoring Companies – Pipeline Products by Stage of Development 24
Therapeutic Drug Monitoring – Pipeline Products by Stage of Development 27
20/20 GeneSystems Inc Pipeline Products & Ongoing Clinical Trials Overview 32
PredicTOR Breast Test – Product Status 32
PredicTOR Breast Test – Product Description 32
Abbott Diagnostics Pipeline Products & Ongoing Clinical Trials Overview 33
Methotrexate Chemiluminescent Assay – Product Status 33
Methotrexate Chemiluminescent Assay – Product Description 33
Adaptive Biotechnologies Corp Pipeline Products & Ongoing Clinical Trials Overview 34
Immune System Profiling – Measure Of Vaccine Efficacy – Product Status 34
Immune System Profiling – Measure Of Vaccine Efficacy – Product Description 34
Affinergy LLC Pipeline Products & Ongoing Clinical Trials Overview 35
Locking Assay – Posaconazole – Product Status 35
Locking Assay – Posaconazole – Product Description 35
Phage-Based Assay – Product Status 36
Phage-Based Assay – Product Description 36
Animated Dynamics Inc Pipeline Products & Ongoing Clinical Trials Overview 37
ONCO4D Assay – Product Status 37
ONCO4D Assay – Product Description 37
Animated Dynamics Inc – Ongoing Clinical Trials Overview 38
ONCO4D Assay – Feasibility Study of Motility Contrast Tomography for Predicting Therapeutic Response 39
ONCO4D Assay – Measuring the Impact of Onco4D Guidance 39
Applied Research using OMIC Sciences SL Pipeline Products & Ongoing Clinical Trials Overview 40
SNP-Test – Product Status 40
SNP-Test – Product Description 40
ARK Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 41
ARK Apixaban Assay – Product Status 41
ARK Apixaban Assay – Product Description 42
ARK Atazanavir Assay – Product Status 42
ARK Atazanavir Assay – Product Description 42
ARK Dabigatran Assay – Product Status 43
ARK Dabigatran Assay – Product Description 43
ARK Darunavir Assay – Product Status 43
ARK Darunavir Assay – Product Description 44
ARK Dolutegravir Assay – Product Status 44
ARK Dolutegravir Assay – Product Description 44
ARK Edoxaban Assay – Product Status 45
ARK Edoxaban Assay – Product Description 45
ARK High Sensitivity Opiates Assay – Product Status 45
ARK High Sensitivity Opiates Assay – Product Description 46
ARK Indinavir Assay – Product Status 46
ARK Indinavir Assay – Product Description 46
ARK Lopinavir Assay – Product Status 47
ARK Lopinavir Assay – Product Description 47
ARK Maraviroc Assay – Product Status 47
ARK Maraviroc Assay – Product Description 48
ARK Nelfinavir Assay – Product Status 48
ARK Nelfinavir Assay – Product Description 48
ARK Nevirapine Assay – Product Status 49
ARK Nevirapine Assay – Product Description 49
ARK Perampanel Assay – Product Status 49
ARK Perampanel Assay – Product Description 50
ARK Rivaroxaban Assay – Product Status 50
ARK Rivaroxaban Assay – Product Description 50
ARK Saquinavir Assay – Product Status 51
ARK Saquinavir Assay – Product Description 51
ARK Tiagabine Assay – Product Status 51
ARK Tiagabine Assay – Product Description 52
ARK Zolpidem Assay – Product Status 52
ARK Zolpidem Assay – Product Description 52
ARK Zopiclone Assay – Product Status 53
ARK Zopiclone Assay – Product Description 53
AutoGenomics Inc Pipeline Products & Ongoing Clinical Trials Overview 54
Plavix Panel Test – Product Status 54
Plavix Panel Test – Product Description 54
Avacta Group Plc Pipeline Products & Ongoing Clinical Trials Overview 55
Depression Test – Product Status 55
Depression Test – Product Description 55
Diagnostic Assay – Alzheimer’s Disease – Product Status 56
Diagnostic Assay – Alzheimer’s Disease – Product Description 56
Diagnostic Test – ADHD – Product Status 56
Diagnostic Test – ADHD – Product Description 57
Diagnostic Test – Bipolar Disorder – Product Status 57
Diagnostic Test – Bipolar Disorder – Product Description 57
Schizophrenia Response Test – Product Status 57
Schizophrenia Response Test – Product Description 58
Schizophrenia Side Effects Test – Product Status 58
Schizophrenia Side Effects Test – Product Description 58
BG Medicine Inc Pipeline Products & Ongoing Clinical Trials Overview 59
Avandia Response – Product Status 59
Avandia Response – Product Description 59
Biomarker Test – Enbrel – Product Status 60
Biomarker Test – Enbrel – Product Description 60
Biomarker Test – Humira – Product Status 60
Biomarker Test – Humira – Product Description 60
Biomarker Test – Remicade – Product Status 61
Biomarker Test – Remicade – Product Description 61
BIOHOPE Scientific SL Pipeline Products & Ongoing Clinical Trials Overview 62
Immunobiogram – Renal Transplantation – Product Status 62
Immunobiogram – Renal Transplantation – Product Description 62
BIOHOPE Scientific SL – Ongoing Clinical Trials Overview 63
Immunobiogram – Renal Transplantation – Longitudinal Observational Study to Determine the Correlation Between Patient’s Pharmacodynamic Response to Immunosuppressants Measured in Vitro with IMMUNOBIOGRAM and of Rejection in Graft Biopsies in Patients with Renal Transplantation 64
Boston University Pipeline Products & Ongoing Clinical Trials Overview 65
ELISA Assay – Tenofovir – Product Status 65
ELISA Assay – Tenofovir – Product Description 65
CCC Diagnostics LLC Pipeline Products & Ongoing Clinical Trials Overview 66
DirectHit Drug Response Indicator Test Panel – Gastrointestinal Cancer – Product Status 66
DirectHit Drug Response Indicator Test Panel – Gastrointestinal Cancer – Product Description 66
Centre Hospitalier Universitaire de Nice Pipeline Products & Ongoing Clinical Trials Overview 67
Azacitidine Resistance Assay – Product Status 67
Azacitidine Resistance Assay – Product Description 67
Chronix Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 68
Therapeutic Monitoring Test – Pancreatic Cancer – Product Status 68
Therapeutic Monitoring Test – Pancreatic Cancer – Product Description 68
Cognizance Biomarkers LLC Pipeline Products & Ongoing Clinical Trials Overview 69
Blood Assay – mTOR Signaling – Product Status 69
Blood Assay – mTOR Signaling – Product Description 69
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 70
Diagnostic Assay – Acetylcholinesterase (AChE) Activity – Product Status 70
Diagnostic Assay – Acetylcholinesterase (AChE) Activity – Product Description 70
ContraVac Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 71
SpermCheck – Contraception – Product Status 71
SpermCheck – Contraception – Product Description 71
Denovo Biolabs Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 72
DeQuanto Trastuzumab (Herceptin) PK ELISA KIT – Product Status 72
DeQuanto Trastuzumab (Herceptin) PK ELISA KIT – Product Description 72
DNAPrint Genomics Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 73
Acenome – Product Status 73
Acenome – Product Description 73
Melphalan/Topotecan Response Test – Product Status 74
Melphalan/Topotecan Response Test – Product Description 74
Statinome – Product Status 74
Statinome – Product Description 75
Dublin City University Pipeline Products & Ongoing Clinical Trials Overview 76
Biomarker Based Test – Multiple Myeloma – Product Status 76
Biomarker Based Test – Multiple Myeloma – Product Description 76
Ecole Polytechnique Federale de Lausanne Pipeline Products & Ongoing Clinical Trials Overview 77
Portable Biosensor – Product Status 77
Portable Biosensor – Product Description 77
ELITechGroup Inc Pipeline Products & Ongoing Clinical Trials Overview 78
Eon 300 Automated Chemistry Analyzer – TxB Cardio Assay – Product Status 78
Eon 300 Automated Chemistry Analyzer – TxB Cardio Assay – Product Description 78
Eon Digoxin Assay – Product Status 79
Eon Digoxin Assay – Product Description 79
Eon Theophylline Assay – Product Status 79
Eon Theophylline Assay – Product Description 79
Phenobarbital Assay – Product Status 80
Phenobarbital Assay – Product Description 80
Phenytoin Assay – Product Status 80
Phenytoin Assay – Product Description 80
Selectra ProM Chemistry System – TxB Cardio Assay – Product Status 81
Selectra ProM Chemistry System – TxB Cardio Assay – Product Description 81
Selectra ProS Chemistry System – TxB Cardio Assay – Product Status 81
Selectra ProS Chemistry System – TxB Cardio Assay – Product Description 82
Eurobio Scientific SA Pipeline Products & Ongoing Clinical Trials Overview 83
Therapeutic Drug Monitoring Test – Resistant Cancer – Product Status 83
Therapeutic Drug Monitoring Test – Resistant Cancer – Product Description 83
Gene Express, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 84
Cisplatin Chemoresistance Test – Product Status 84
Cisplatin Chemoresistance Test – Product Description 84
Prognostic Test – Irinotecan Chemoresistance – Product Status 85
Prognostic Test – Irinotecan Chemoresistance – Product Description 85
GenMark Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 86
2D6 Tamoxifen Metabolism Test – Product Status 86
2D6 Tamoxifen Metabolism Test – Product Description 86
eSensor 2C19 Genotyping Test – Product Status 87
eSensor 2C19 Genotyping Test – Product Description 87
Horiba Ltd Pipeline Products & Ongoing Clinical Trials Overview 88
ABX Pentra 400 Analyzer – TDM Assay – Product Status 88
ABX Pentra 400 Analyzer – TDM Assay – Product Description 88
Pentra C200 Analyzer – TDM Assay – Product Status 89
Pentra C200 Analyzer – TDM Assay – Product Description 89
Inflammatix Inc Pipeline Products & Ongoing Clinical Trials Overview 90
HostDxTNFresponse – Product Status 90
HostDxTNFresponse – Product Description 90
Instrumentation Laboratory Co Pipeline Products & Ongoing Clinical Trials Overview 91
HemosIL Dabigatran Assay – Product Status 91
HemosIL Dabigatran Assay – Product Description 91
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 92
Predictive Test For Chemotherapy Regimen – Product Status 92
Predictive Test For Chemotherapy Regimen – Product Description 92
KIYATEC Inc Pipeline Products & Ongoing Clinical Trials Overview 93
Ex Vivo 3D Drug Response (EV3D) Assay – Product Status 93
Ex Vivo 3D Drug Response (EV3D) Assay – Product Description 93
KIYATEC Inc – Ongoing Clinical Trials Overview 94
Ex Vivo 3D Drug Response (EV3D) Assay – 3D Prediction of Patient-specific Response Using Ex Vivo Interrogation of Live Cells from Tumors: 3D-PREDICT REGISTRY 95
Medibio Ltd Pipeline Products & Ongoing Clinical Trials Overview 96
Medibio RX – Bipolar – Product Status 96
Medibio RX – Bipolar – Product Description 96
Medibio RX – General Anxiety Disorders – Product Status 97
Medibio RX – General Anxiety Disorders – Product Description 97
Medibio RX – PTSD – Product Status 97
Medibio RX – PTSD – Product Description 97
Medibio RX – Schizophrenia – Product Status 98
Medibio RX – Schizophrenia – Product Description 98
Medical Research Council Pipeline Products & Ongoing Clinical Trials Overview 99
Diagnostic Assay – Hormone Dependent Tumours – Product Status 99
Diagnostic Assay – Hormone Dependent Tumours – Product Description 99
Metabolon Inc Pipeline Products & Ongoing Clinical Trials Overview 100
Cisplatin Tolerance Test – Product Status 100
Cisplatin Tolerance Test – Product Description 100
Prostate Cancer TDM Test – Product Status 101
Prostate Cancer TDM Test – Product Description 101
Newcastle University Pipeline Products & Ongoing Clinical Trials Overview 102
Thiopurines Assay – Product Status 102
Thiopurines Assay – Product Description 102
Nipro Europe N.V. Pipeline Products & Ongoing Clinical Trials Overview 103
Lithium Assay – Product Status 103
Lithium Assay – Product Description 103
Nuclea Biotechnologies Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 104
EGFR TK Sensitivity Lung Cancer Diagnostic Test – Product Status 104
EGFR TK Sensitivity Lung Cancer Diagnostic Test – Product Description 104
Irinotecan Sensitivity Diagnostic Test – Colon Cancer – Product Status 105
Irinotecan Sensitivity Diagnostic Test – Colon Cancer – Product Description 105
PanGenomic Health Inc Pipeline Products & Ongoing Clinical Trials Overview 106
Treatment Efficacy Monitoring Assay – Product Status 106
Treatment Efficacy Monitoring Assay – Product Description 106
Pax Neuroscience Inc Pipeline Products & Ongoing Clinical Trials Overview 107
MoodMark Rx – Product Status 107
MoodMark Rx – Product Description 107
Pelago Bioscience AB Pipeline Products & Ongoing Clinical Trials Overview 108
CETSA – Product Status 108
CETSA – Product Description 108
Pennsylvania State University Pipeline Products & Ongoing Clinical Trials Overview 109
Antiobody-Based ELISA Assay – ACTS – Product Status 109
Antiobody-Based ELISA Assay – ACTS – Product Description 109
Plateletdiagnostics LLC Pipeline Products & Ongoing Clinical Trials Overview 110
pDrp1 Assay – Product Status 110
pDrp1 Assay – Product Description 110
Prediction Sciences LLC (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 111
GeneRx For Hypertension Treatment – Product Status 111
GeneRx For Hypertension Treatment – Product Description 111
GeneRx Lithium/Bipolar Test – Product Status 112
GeneRx Lithium/Bipolar Test – Product Description 112
ProciseDx LLC Pipeline Products & Ongoing Clinical Trials Overview 113
Procise ADL – Product Status 113
Procise ADL – Product Description 113
Procise IFX – Product Status 114
Procise IFX – Product Description 114
Progenika Biopharma SA Pipeline Products & Ongoing Clinical Trials Overview 115
Promonitor – Autoimmune And Inflammation – Product Status 115
Promonitor – Autoimmune And Inflammation – Product Description 115
PrognosDx Health Inc Pipeline Products & Ongoing Clinical Trials Overview 116
RxScore Therapeutic Response Test – Breast Cancer – Product Status 116
RxScore Therapeutic Response Test – Breast Cancer – Product Description 116
RxScore Therapeutic Response Test – Pancreatic Cancer – Product Status 117
RxScore Therapeutic Response Test – Pancreatic Cancer – Product Description 117
Prometheus Laboratories Inc Pipeline Products & Ongoing Clinical Trials Overview 118
PL-200 PredictrPK – CD/UC Adalimumab – Product Status 118
PL-200 PredictrPK – CD/UC Adalimumab – Product Description 119
PL-200 PredictrPK – CD/UC Infliximab – Product Status 119
PL-200 PredictrPK – CD/UC Infliximab – Product Description 120
PL-200 PredictrPK – CD/UC Ustekinumab – Product Status 120
PL-200 PredictrPK – CD/UC Ustekinumab – Product Description 121
PL-200 PredictrPK – CD/UC Vedolizumab – Product Status 121
PL-200 PredictrPK – CD/UC Vedolizumab – Product Description 121
PL-300 Monitr – Product Status 122
PL-300 Monitr – Product Description 122
PL-400 CD/UC Infliximab/Adalimumab – Product Status 122
PL-400 CD/UC Infliximab/Adalimumab – Product Description 123
PL-600 Anser CD Risankizumab – Product Status 123
PL-600 Anser CD Risankizumab – Product Description 123
Proteomika (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 124
Promonitor-Anti-CZP (Certolizumab) – Product Status 124
Promonitor-Anti-CZP (Certolizumab) – Product Description 124
Promonitor-Anti-TCZ (Tocilizumab) – Product Status 125
Promonitor-Anti-TCZ (Tocilizumab) – Product Description 125
Promonitor-CZP (Certolizumab) – Product Status 125
Promonitor-CZP (Certolizumab) – Product Description 126
Promonitor-TCZ (Tocilizumab) – Product Status 126
Promonitor-TCZ (Tocilizumab) – Product Description 126
Purdue University Pipeline Products & Ongoing Clinical Trials Overview 127
Point-Of-Care Mass Spectrometry Analysis System – Product Status 127
Point-Of-Care Mass Spectrometry Analysis System – Product Description 127
Queen Mary University of London Pipeline Products & Ongoing Clinical Trials Overview 128
Hepatocyte – Based Drug Screen Assay – Product Status 128
Hepatocyte – Based Drug Screen Assay – Product Description 128
Roche Diagnostics Corp Pipeline Products & Ongoing Clinical Trials Overview 129
Liat Assay – Pharmacogenomics – Product Status 129
Liat Assay – Pharmacogenomics – Product Description 129
Roche Diagnostics International Ltd Pipeline Products & Ongoing Clinical Trials Overview 130
Cobas – Cyclosporin A Assay – Product Status 131
Cobas – Cyclosporin A Assay – Product Description 131
Cobas 6000 Analyzer Series With c 501 Module – Cyclosporine Assay – Product Status 132
Cobas 6000 Analyzer Series With c 501 Module – Cyclosporine Assay – Product Description 132
Cobas 6000 Analyzer Series With c 501 Module – Lidocaine Assay – Product Status 132
Cobas 6000 Analyzer Series With c 501 Module – Lidocaine Assay – Product Description 132
Cobas 6000 Analyzer Series With c 501 Module – Tacrolimus Assay – Product Status 133
Cobas 6000 Analyzer Series With c 501 Module – Tacrolimus Assay – Product Description 133
Cobas 6000 Analyzer Series With e 601 Module – Cyclosporine Assay – Product Status 133
Cobas 6000 Analyzer Series With e 601 Module – Cyclosporine Assay – Product Description 134
Cobas 6000 Analyzer Series With e 601 Module – Everolimus Assay – Product Status 134
Cobas 6000 Analyzer Series With e 601 Module – Everolimus Assay – Product Description 134
Cobas 6000 Analyzer Series With e 601 Module – Sirolimus Assay – Product Status 135
Cobas 6000 Analyzer Series With e 601 Module – Sirolimus Assay – Product Description 135
Cobas 8000 Analyzer Series With c 502 Module – Everolimus Assay – Product Status 135
Cobas 8000 Analyzer Series With c 502 Module – Everolimus Assay – Product Description 136
Cobas 8000 Analyzer Series With c 502 Module – Lidocaine Assay – Product Status 136
Cobas 8000 Analyzer Series With c 502 Module – Lidocaine Assay – Product Description 136
Cobas 8000 Analyzer Series With c 502 Module – Sirolimus Assay – Product Status 136
Cobas 8000 Analyzer Series With c 502 Module – Sirolimus Assay – Product Description 137
Cobas 8000 Analyzer Series With c 502 Module – Tacrolimus Assay – Product Status 137
Cobas 8000 Analyzer Series With c 502 Module – Tacrolimus Assay – Product Description 137
Cobas 8000 Analyzer Series With c 701 Module – Everolimus Assay – Product Status 138
Cobas 8000 Analyzer Series With c 701 Module – Everolimus Assay – Product Description 138
Cobas 8000 Analyzer Series With c 701 Module – Lidocaine Assay – Product Status 138
Cobas 8000 Analyzer Series With c 701 Module – Lidocaine Assay – Product Description 139
Cobas 8000 Analyzer Series With c 701 Module – Sirolimus Assay – Product Status 139
Cobas 8000 Analyzer Series With c 701 Module – Sirolimus Assay – Product Description 139
Cobas 8000 Analyzer Series With c 701 Module – Tacrolimus Assay – Product Status 140
Cobas 8000 Analyzer Series With c 701 Module – Tacrolimus Assay – Product Description 140
Cobas 8000 Analyzer Series With c 702 Module – Everolimus Assay – Product Status 140
Cobas 8000 Analyzer Series With c 702 Module – Everolimus Assay – Product Description 141
Cobas 8000 Analyzer Series With c 702 Module – Lidocaine Assay – Product Status 141
Cobas 8000 Analyzer Series With c 702 Module – Lidocaine Assay – Product Description 141
Cobas 8000 Analyzer Series With c 702 Module – Sirolimus Assay – Product Status 142
Cobas 8000 Analyzer Series With c 702 Module – Sirolimus Assay – Product Description 142
Cobas 8000 Analyzer Series With c 702 Module – Tacrolimus Assay – Product Status 142
Cobas 8000 Analyzer Series With c 702 Module – Tacrolimus Assay – Product Description 143
Cobas 8000 Analyzer Series With e 602 Module – Everolimus Assay – Product Status 143
Cobas 8000 Analyzer Series With e 602 Module – Everolimus Assay – Product Description 143
Cobas 8000 Analyzer Series With e 602 Module – Lidocaine Assay – Product Status 144
Cobas 8000 Analyzer Series With e 602 Module – Lidocaine Assay – Product Description 144
Cobas 8000 Analyzer Series With e 801 Module – Everolimus Assay – Product Status 144
Cobas 8000 Analyzer Series With e 801 Module – Everolimus Assay – Product Description 144
Cobas 8000 Analyzer Series With e 801 Module – Tacrolimus Assay – Product Status 145
Cobas 8000 Analyzer Series With e 801 Module – Tacrolimus Assay – Product Description 145
Cobas t 511 Coagulation Analyzer – Apixaban Assay – Product Status 145
Cobas t 511 Coagulation Analyzer – Apixaban Assay – Product Description 146
Cobas t 511 Coagulation Analyzer – Dabigatran Assay – Product Status 146
Cobas t 511 Coagulation Analyzer – Dabigatran Assay – Product Description 146
Cobas t 511 Coagulation Analyzer – Rivaroxaban Assay – Product Status 147
Cobas t 511 Coagulation Analyzer – Rivaroxaban Assay – Product Description 147
Cobas t 711 Coagulation Analyzer – Apixaban Assay – Product Status 147
Cobas t 711 Coagulation Analyzer – Apixaban Assay – Product Description 147
Cobas t 711 Coagulation Analyzer – Dabigatran Assay – Product Status 148
Cobas t 711 Coagulation Analyzer – Dabigatran Assay – Product Description 148
Cobas t 711 Coagulation Analyzer – Rivaroxaban Assay – Product Status 148
Cobas t 711 Coagulation Analyzer – Rivaroxaban Assay – Product Description 148
Integrated Modular Analytics – Tacrolimus Test – Product Status 149
Integrated Modular Analytics – Tacrolimus Test – Product Description 149
Integrated Modular Analytics System – Mycophenolic Acid Test – Product Status 149
Integrated Modular Analytics System – Mycophenolic Acid Test – Product Description 149
Rockland Immunochemicals Inc Pipeline Products & Ongoing Clinical Trials Overview 150
Akt/mTOR Signaling Pharmacodynamics Assay – Product Status 150
Akt/mTOR Signaling Pharmacodynamics Assay – Product Description 150
SAGA Diagnostics AB Pipeline Products & Ongoing Clinical Trials Overview 151
IBSAFE – Therapy Response Monitoring & Drug Resistance – Product Status 151
IBSAFE – Therapy Response Monitoring & Drug Resistance – Product Description 151
KROMA – Therapy Response Monitoring & Drug Resistance – Product Status 152
KROMA – Therapy Response Monitoring & Drug Resistance – Product Description 152
Saladax Biomedical Inc Pipeline Products & Ongoing Clinical Trials Overview 153
MyCare Assay – Busulfan – Product Status 153
MyCare Assay – Busulfan – Product Description 154
MyCare Assay – Cyclophosphamide – Product Status 154
MyCare Assay – Cyclophosphamide – Product Description 154
MyCare Assay – Doxorubicin – Product Status 154
MyCare Assay – Doxorubicin – Product Description 155
MyCare Assay – Gemcitabine – Product Status 155
MyCare Assay – Gemcitabine – Product Description 155
MyCare Assay – Irinotecan – Product Status 156
MyCare Assay – Irinotecan – Product Description 156
MyCare Assay – Lenalidomide – Product Status 156
MyCare Assay – Lenalidomide – Product Description 156
MyCare Assay – Thalidomide – Product Status 157
MyCare Assay – Thalidomide – Product Description 157
MyCare Assay – Tyrosine Kinase Inhibitor – Product Status 157
MyCare Assay – Tyrosine Kinase Inhibitor – Product Description 157
MyCare Assay – Vincristine – Product Status 158
MyCare Assay – Vincristine – Product Description 158
MyCare Psychiatry Aripiprazole Assay Kit – Product Status 158
MyCare Psychiatry Aripiprazole Assay Kit – Product Description 159
MyCare Psychiatry Olanzapine Assay Kit – Product Status 159
MyCare Psychiatry Olanzapine Assay Kit – Product Description 159
MyCare Psychiatry Quetiapine Assay Kit – Product Status 160
MyCare Psychiatry Quetiapine Assay Kit – Product Description 160
MyCare Psychiatry Total Risperidone Assay Kit – Product Status 160
MyCare Psychiatry Total Risperidone Assay Kit – Product Description 161
Nanoparticle Immunoassay Kit – TDM – Product Status 161
Nanoparticle Immunoassay Kit – TDM – Product Description 161
Saladax Biomedical Inc – Ongoing Clinical Trials Overview 162
MyCare Assay – Busulfan – Collection of Plasma Samples Using Sodium Heparin From Subjects Undergoing Intravenous Busulfan Treatment 163
Siemens Healthcare Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 164
ADVIA Everolimus Assay – Product Status 164
ADVIA Everolimus Assay – Product Description 164
ADVIA Sirolimus Assay – Product Status 165
ADVIA Sirolimus Assay – Product Description 165
ADVIA Tacrolimus Assay – Product Status 165
ADVIA Tacrolimus Assay – Product Description 166
Atellica IM Analyzer – Everolimus Assay – Product Status 166
Atellica IM Analyzer – Everolimus Assay – Product Description 166
Atellica IM Analyzer – Sirolimus Assay – Product Status 167
Atellica IM Analyzer – Sirolimus Assay – Product Description 167
Atellica Solution Clinical Chemistry Analyzer – Levetiracetam – Product Status 167
Atellica Solution Clinical Chemistry Analyzer – Levetiracetam – Product Description 168
Sirius Genomics Inc Pipeline Products & Ongoing Clinical Trials Overview 169
Pharmacogenetic Test – Product Status 169
Pharmacogenetic Test – Product Description 169
Spanish National Research Council Pipeline Products & Ongoing Clinical Trials Overview 170
Blood-Based Immunoassay – Oral Anticoagulants – Product Status 170
Blood-Based Immunoassay – Oral Anticoagulants – Product Description 170
SQI Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 171
Ig_PLEX TNF Assay – Product Status 171
Ig_PLEX TNF Assay – Product Description 171
Multiplex Anti Drug Antibody Assay – Product Status 172
Multiplex Anti Drug Antibody Assay – Product Description 172
T2 Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview 173
T2Endotoxin – Product Status 173
T2Endotoxin – Product Description 173
Thermo Fisher Scientific Inc Pipeline Products & Ongoing Clinical Trials Overview 174
Cascadion SM Clinical Analyzer – Therapeutic Drug Monitoring – Product Status 174
Cascadion SM Clinical Analyzer – Therapeutic Drug Monitoring – Product Description 174
Cascadion SM Immunosuppressants Panel – Product Status 175
Cascadion SM Immunosuppressants Panel – Product Description 175
Universidade Feevale Pipeline Products & Ongoing Clinical Trials Overview 176
Dried Plasma Spot Assay – Therapeutic Drug Monitoring – Product Status 176
Dried Plasma Spot Assay – Therapeutic Drug Monitoring – Product Description 176
University of Colorado Pipeline Products & Ongoing Clinical Trials Overview 177
Biomarker Assay – Schizophrenia – Product Status 177
Biomarker Assay – Schizophrenia – Product Description 177
Pharmacokinetic Drug Monitoring Device – Product Status 178
Pharmacokinetic Drug Monitoring Device – Product Description 178
University of Colorado Denver Pipeline Products & Ongoing Clinical Trials Overview 179
Therapeutic Drug Monitoring Test – HIV – Product Status 179
Therapeutic Drug Monitoring Test – HIV – Product Description 179
University of North Dakota Pipeline Products & Ongoing Clinical Trials Overview 180
Screening Assay – Anti Viral Drug – Product Status 180
Screening Assay – Anti Viral Drug – Product Description 180
University of Queensland Pipeline Products & Ongoing Clinical Trials Overview 181
Theranostic Assay – Squamous Cell Carcinoma – Product Status 181
Theranostic Assay – Squamous Cell Carcinoma – Product Description 181
University of Washington Pipeline Products & Ongoing Clinical Trials Overview 182
Point-Of-Care Assay – Tenofovir – Product Status 182
Point-Of-Care Assay – Tenofovir – Product Description 182
Vyant Bio Inc Pipeline Products & Ongoing Clinical Trials Overview 183
Comprehensive Pharmacogenomics Panel Test – Product Status 183
Comprehensive Pharmacogenomics Panel Test – Product Description 183
Glossary 258
![]()