Tissue Sealants Pipeline by Development Stages, Segments, Region and Countries, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Tissue Sealants Pipeline Market Report Overview
A tissue sealant serves as a physical barrier to prevent leakage of fluid and/or gas from a surgical incision. These sealants can be used to hold the flaps of internal organs together, to seal the blood vessels in injury or surgery, or as an adjunct with hemostatic agents. The volume of tissue sealant used per procedure varies depending on the type of procedure and formulation.
The Tissue Sealants pipeline market research report provides a comprehensive understanding of the pipeline products along with their comparative analysis at various stages of development. The report also includes information about the territories wherein the clinical trials are in progress, the regulatory paths followed by the trials, and the companies associated with the trials.
| Key Segments | · Synthetic Tissue Sealants
· Fibrin Based Tissue Sealants · Other Protein-Based Tissue Sealants · Combination Hemostats – Flowables · Fibrin Based Tissue Sealants in Liquid Form |
| Key Territories | · The US
· Europe · Canada · China · Australia |
| Key Regulatory Paths | · 510(K)
· CE · TGA · MDL · NMPA |
| Leading Companies | · Advanced Medical Solutions Group plc
· Aleo BME Inc · Angiotech Pharmaceuticals Inc · Arch Therapeutics Inc · Arizona State University |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Tissue Sealants Pipeline Market by Segments
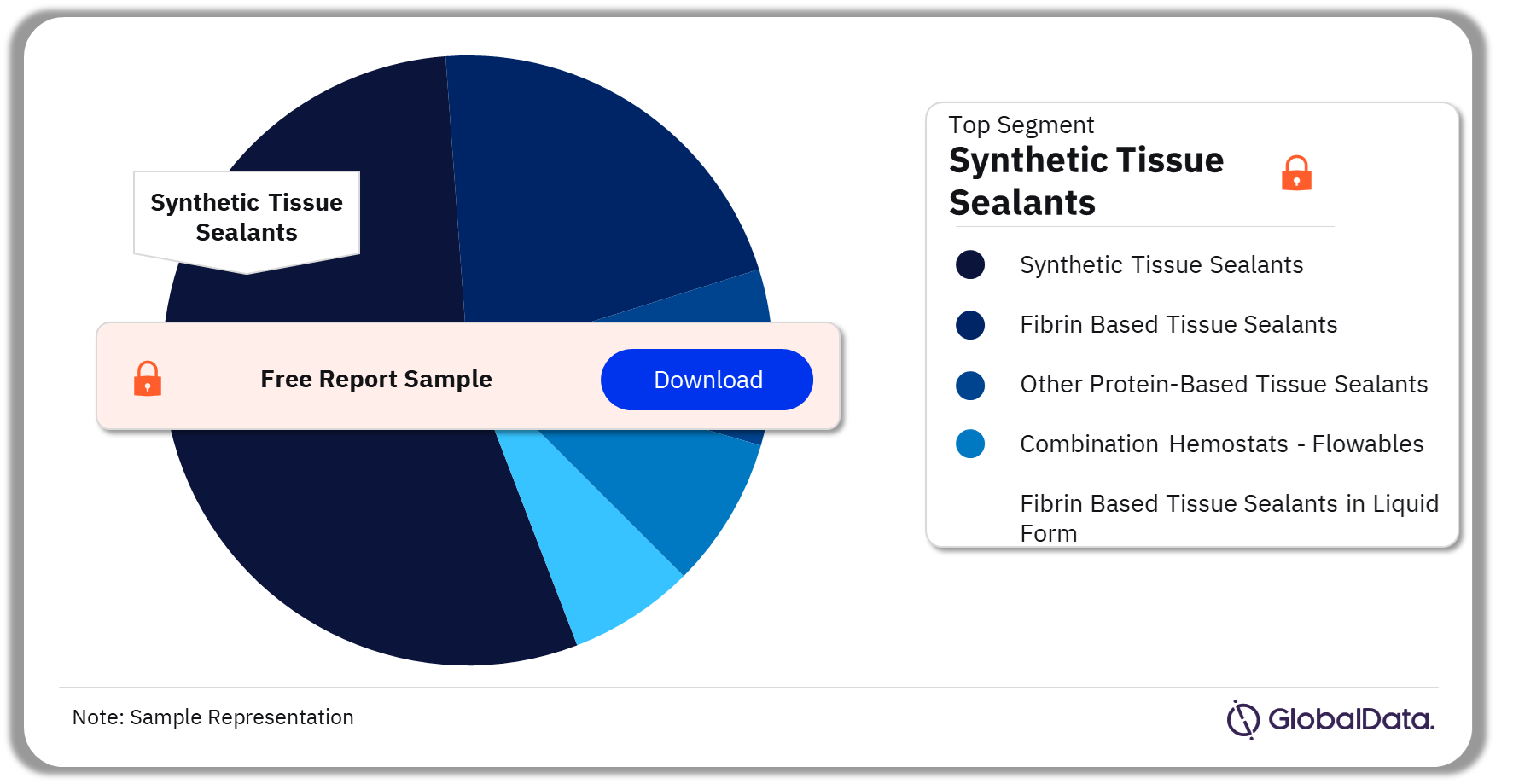
The key segments in the Tissue Sealants pipeline market are Synthetic Tissue Sealants, Fibrin Based Tissue Sealants, Other Protein-Based Tissue Sealants, Combination Hemostats – Flowables, and Fibrin Based Tissue Sealants in Liquid Form. As of December 2023, Synthetic Tissue Sealants accounted for the highest number of pipeline products. Synthetic Tissue Sealants are made of chemical compounds that consist of a variety of artificial components. Polyethylene glycol, synthetic peptides, and protein polymers are a few examples of the artificial components used in the manufacturing of Synthetic Tissue Sealants.
Tissue Sealants Pipeline Market Analysis by Segments, 2023 (%)
Buy the Full Report for More Segment Insights into the Tissue Sealants Pipeline Market
Tissue Sealants Pipeline Market Segmentation by Territories
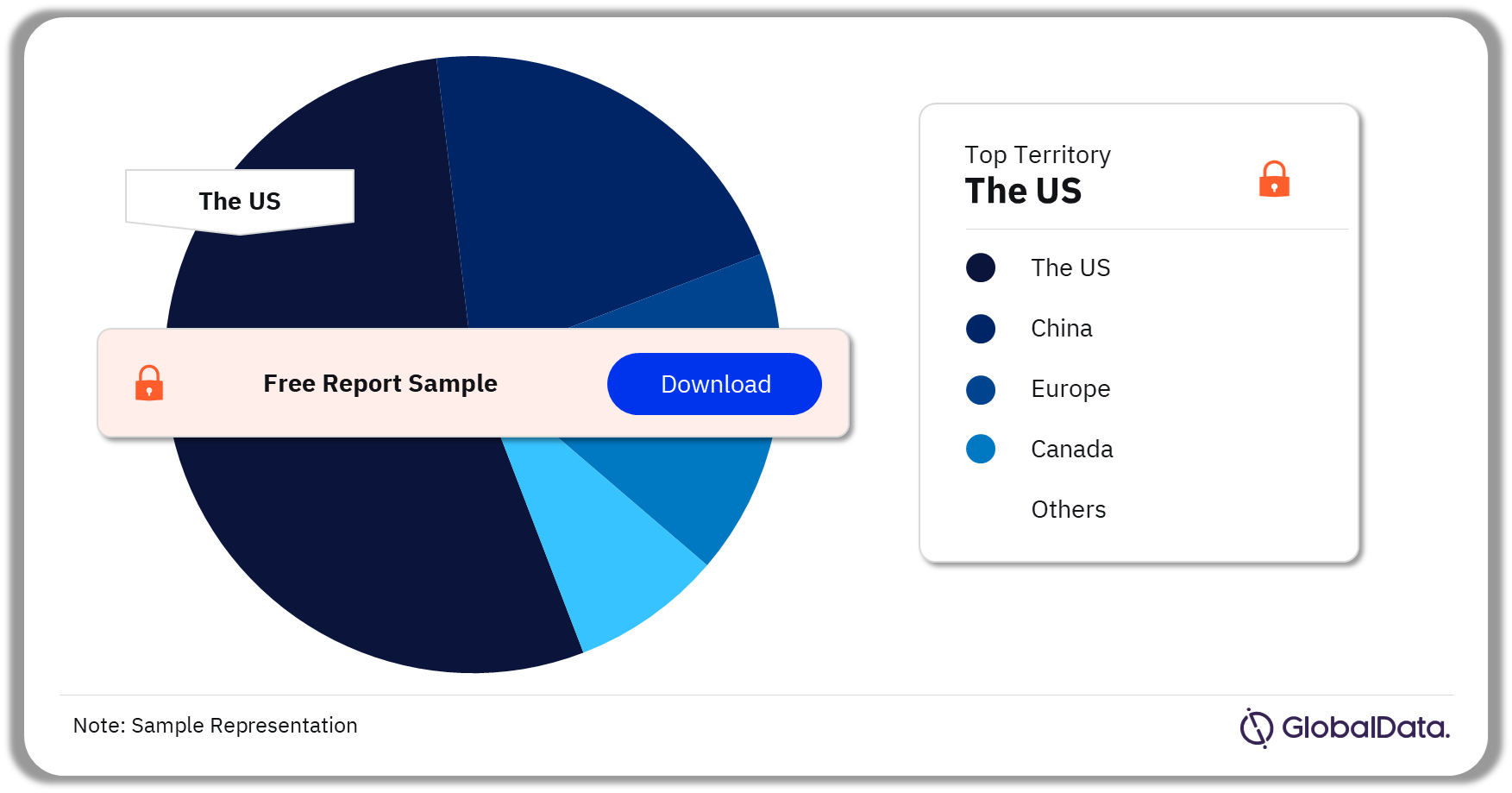
A few of the key territories for Tissue Sealants pipeline products are the US, Europe, Canada, China, and Australia among others. As of December 2023, the US accounted for the highest number of pipeline products.
Tissue Sealants Pipeline Market Analysis by Territories, 2023 (%)
Buy the Full Report for More Territory Insights into the Tissue Sealants Pipeline Market
Tissue Sealants Pipeline Market Segmentation by Regulatory Paths
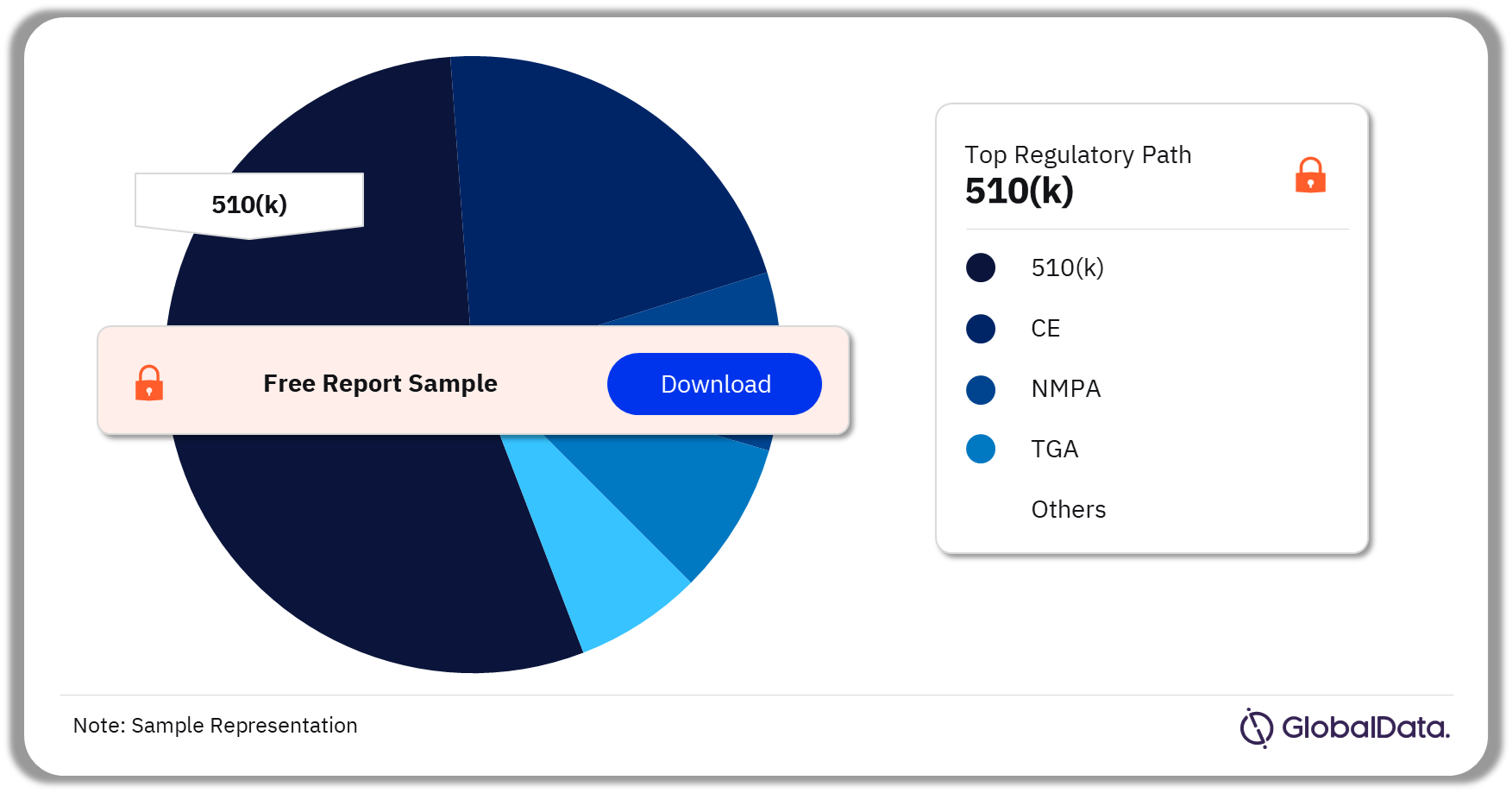
A few of the key regulatory paths followed by the Tissue Sealants pipeline market are 510(K), CE, TGA, MDL, and NMPA among others. As of December 2023, 510(K) was the most followed pathway for pipeline products in the market.
Tissue Sealants Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy the Full Report for More Regulatory Path Insights into the Tissue Sealants Pipeline Market
Tissue Sealants Pipeline Market – Competitive Landscape
A few of the key companies associated with the Tissue Sealants pipeline market are Advanced Medical Solutions Group plc, Aleo BME Inc., Angiotech Pharmaceuticals Inc., Arch Therapeutics Inc., and Arizona State University among others.
Advanced Medical Solutions Group plc (AMS): AMS is headquartered in Winsford, Cheshire, the UK. The company designs, manufactures and distributes wound care dressings and medical adhesives for closing wounds and sealing tissues. The company’s products include tissue adhesives, collagen, sutures, oxidized cellulose, internal fixation, alginate sealants, and antimicrobial dressings among others. These products are distributed through major wound care partners and private-label distributors. Apart from its operations in the UK, the company also has a strong presence in the Netherlands, France, Germany, the Czech Republic, Israel, and Russia.
Tissue Sealants Pipeline Market Analysis by Companies, 2023
Buy the Full Report for More Company Insights into the Tissue Sealants Pipeline Market
Segments Covered in the Report
Tissue Sealants Pipeline Market Segments Outlook
- Synthetic Tissue Sealants
- Fibrin Based Tissue Sealants
- Other Protein-Based Tissue Sealants
- Combination Hemostats – Flowables
- Fibrin Based Tissue Sealants in Liquid Form
Tissue Sealants Pipeline Market Territories Outlook
- The US
- Europe
- Canada
- China
- Australia
Tissue Sealants Pipeline Market Regulatory Paths Outlook
- 510(K)
- CE
- TGA
- MDL
- NMPA
Scope
This report provides:
- Extensive coverage of the Tissue Sealants under development.
- An exhaustive list of major pipeline products and their details, including product descriptions, licensing and collaboration details, and other developmental activities.
- Reviews of major players involved in the development of Tissue Sealants and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report will enable you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies.
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage.
- Identify and understand important and diverse types of Tissue Sealants under development.
- Develop market-entry and market-expansion strategies.
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline.
- Carry out In-depth analysis of the product’s current stage of development, territory, and estimated launch date.
Aleo BME Inc
Angiotech Pharmaceuticals Inc
Arch Therapeutics Inc
Arizona State University
Baxter International Inc
Biomedica Management Corp
Boston University
Calmare Therapeutics Inc
Clemson University
Cohera Medical Inc
Covalent Medical, Inc. (Inactive)
CR Bard Inc
Department of Biomedical Engineering Columbia University
DSM Biomedical BV
Eidgenossische Materialprufungs- und Forschungsanstalt
Elastagen Pty Ltd
Ethicon Endo-Surgery Inc
Gamma Diagnostics Inc
GATT Technologies BV
Hemostasis LLC
HyperBranch Medical Technology Inc
Innotherapy Inc
Institute of Molecular Biology of Parana
InVivo Therapeutics Corp
Johns Hopkins University
Kollodis BioSciences Inc
Kuros Biosciences AG
Laser Tissue Welding, Inc.
Medisse BV (Inactive)
Meril Life Sciences Pvt Ltd
Nurami Medical Ltd
Pharming Group NV
Polyganics BV
Protein Polymer Technologies Inc (Inactive)
Purdue University
Samyang Biopharmaceuticals Corp
Sea Run Holdings Inc
Sealantis Ltd.
Sealonix Inc
Selio Medical
Shanghai Haohai Biological Technology Co Ltd
Surgical Sealants, Inc.
Teijin Pharma Ltd
Terasaki Institute for Biomedical Innovation
Tetratherix Pty Ltd
Texas A&M Engineering Experiment Station
Texas A&M University
The Medicines Co
Tissium SA
University of Akron
Table of Contents
Table
Figures
Frequently asked questions
-
Which was the leading segment in the Tissue Sealants pipeline market?
Synthetic Tissue Sealants was the leading segment in the Tissue Sealants pipeline market as of December 2023.
-
Which territory had the highest number of products in the Tissue Sealants pipeline market?
As of December 2023, the US accounted for the highest number of products in the Tissue Sealants pipeline market.
-
Which was the most followed regulatory pathway in the Tissue Sealants pipeline market?
As of December 2023, 510(K) was the most followed pathway for pipeline products in the Tissue Sealants market.
-
Which are the major companies operating in the Tissue Sealants pipeline market?
A few of the key companies associated with the Tissue Sealants pipeline market are Advanced Medical Solutions Group plc, Aleo BME Inc., Angiotech Pharmaceuticals Inc., Arch Therapeutics Inc., and Arizona State University among others.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Tissue Sealants reports