Topical Absorbable Hemostats Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Topical absorbable hemostats are agents used to promote and accelerate hemostasis to control bleeding in cases where application of pressure, ligature and other conventional methods is ineffective. They are inserted to remove excess blood, thereby preventing further bleeding and clearing up the operating site for the surgeon.
The topical absorbable hemostats market research report reviews details of major pipeline products which includes, product description, licensing and collaboration details, and other developmental activities. The report also reviews the major players involved in the development of topical absorbable hemostats and lists all their pipeline projects.
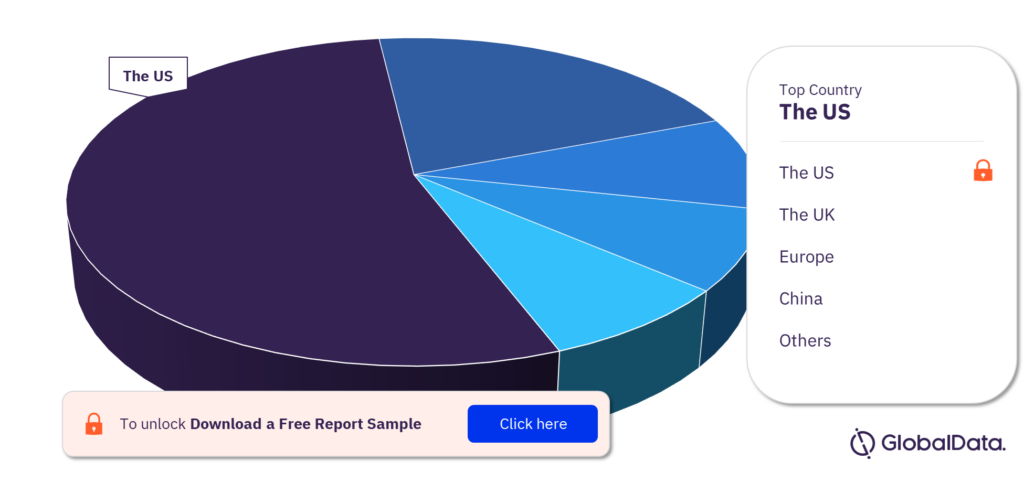
Which are the key territories in the topical absorbable hemostats pipeline market?
The key territories in the topical absorbable hemostats pipeline market are the United States, Europe, India, South Korea, China, Japan, Canada, Russia, Singapore, Taiwan, the United Kingdom, Vietnam, Australia, and Philippines. The United States has the highest number of pipeline products.
Topical absorbable hemostats pipeline market, by territories
For more territory insights, download a free report sample
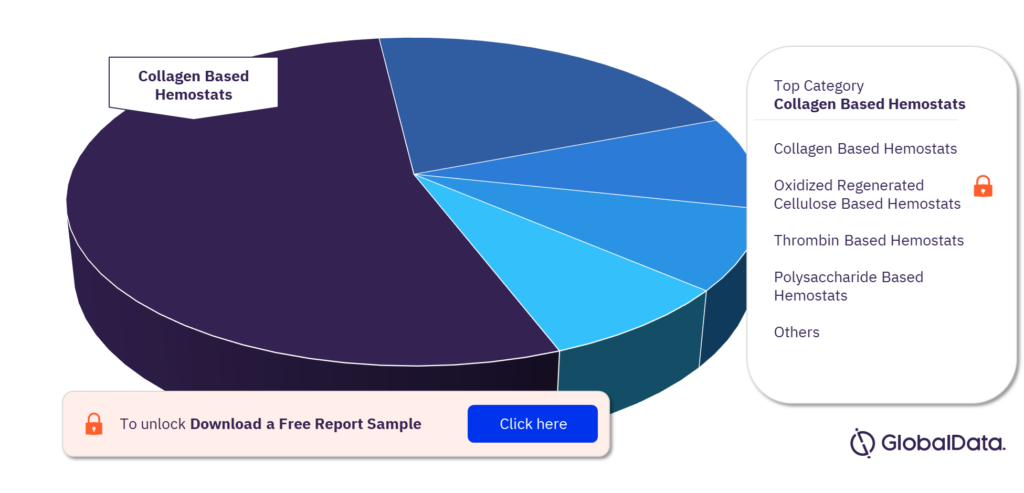
Which are the key categories in the topical absorbable hemostats pipeline market?
The key categories in the topical absorbable hemostats pipeline market are Collagen Based Hemostats, Oxidized Regenerated Cellulose Based Hemostats, Thrombin Based Hemostats, Polysaccharide Based Hemostats, Combination Hemostats – Pads, and Gelatin Based Hemostats. Collagen Based Hemostats has the highest number of pipeline products.
Topical absorbable hemostats pipeline market, by categories
For more category insights, download a free report sample
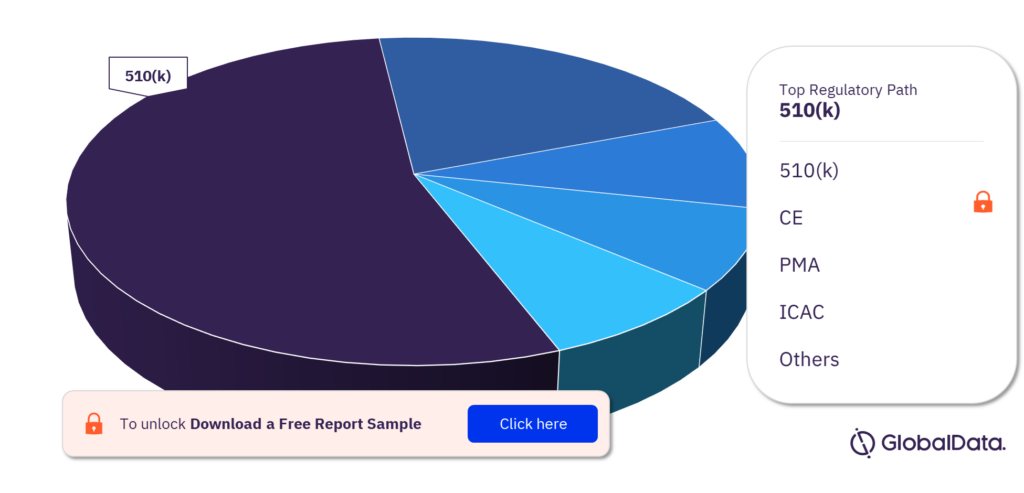
Which are the key regulatory paths in the topical absorbable hemostats pipeline market?
The key regulatory paths in the topical absorbable hemostats pipeline market are 510(k), CE, PMA, ICAC, NMPA, MDITAC, MDL, NIFDS, Ninsho, HSA, BOPA, Shonin, TGA, and UKCA. 510(k) has the highest number of pipeline products.
Topical absorbable hemostats pipeline market, by companies
For more regulatory path insights, download a free report sample
Which are the key companies in the topical absorbable hemostats pipeline market?
The key companies in the topical absorbable hemostats pipeline market are 3-D Matrix Ltd, Acro Biomedical Co Ltd, Advanced Biotech Products Pvt Ltd, Angiotech Pharmaceuticals Inc, Anika Therapeutics Inc, APRUS Bio-Medical Innovations Pvt Ltd, Arch Therapeutics Inc, Baxter Healthcare Corp, Beth Israel Deaconess Medical Center, Biomedica Management Corp, Biom’Up SAS, and Cellphire Inc.
Market report overview
| Key territories | The United States, Europe, India, South Korea, China, Japan, Canada, Russia, Singapore, Taiwan, the United Kingdom, Vietnam, Australia, and Philippines |
| Key categories | Collagen Based Hemostats, Oxidized Regenerated Cellulose Based Hemostats, Thrombin Based Hemostats, Polysaccharide Based Hemostats, Combination Hemostats – Pads, and Gelatin Based Hemostats |
| Key regulatory paths | 510(k), CE, PMA, ICAC, NMPA, MDITAC, MDL, NIFDS, Ninsho, HSA, BOPA, Shonin, TGA, and UKCA |
| Key companies | 3-D Matrix Ltd, Acro Biomedical Co Ltd, Advanced Biotech Products Pvt Ltd, Angiotech Pharmaceuticals Inc, Anika Therapeutics Inc, APRUS Bio-Medical Innovations Pvt Ltd, Arch Therapeutics Inc, Baxter Healthcare Corp, Beth Israel Deaconess Medical Center, Biomedica Management Corp, Biom’Up SAS, and Cellphire Inc |
Scope
- Extensive coverage of the Topical Absorbable Hemostats under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- The report reviews the major players involved in the development of Topical Absorbable Hemostats and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Topical Absorbable Hemostats under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory and estimated launch date
Advamedica Inc
Angiotech Pharmaceuticals Inc
Anika Therapeutics Inc
APRUS Bio-Medical Innovations Pvt Ltd
Arch Therapeutics Inc
Baxter Healthcare Corp
Beth Israel Deaconess Medical Center
Biomedica Management Corp
Biom'Up SAS
Cellphire Inc
Ceramicos Para Aplicacoes Medicas SA
Columbia University
Covalent Medical, Inc. (Inactive)
Covalon Technologies Ltd
CR Bard Inc
Cresilon Inc
Endomedix Incorporated
Entegrion Inc
Ethicon US LLC
ETX Pharma Inc
Gamma Therapeutics Inc
GATT Technologies BV
Haemostatix Ltd
Hemostasis LLC
HLL Lifecare Ltd
Ichor Sciences LLC
Keratin Biosciences Inc
Leader Biomedical Europe BV
Medcura Inc
Protege Biomedical LLC
Resorba GmbH
Rice University
Sanofi Biosurgery Inc
Sea Run Holdings Inc
St Teresa Medical Inc
STB Lifesaving Technologies
Tectum
The Medicines Co
Therus Corporation
Thrombotargets Corp
United Health Products, Inc.
University of Grenoble Alpes
Virginia Commonwealth University
Xcede Technologies Inc
Z-Medica LLC
Table of Contents
Table
Figures
Frequently asked questions
-
Which are the key territories in the topical absorbable hemostats pipeline market?
The key territories in the topical absorbable hemostats pipeline market are the United States, Europe, India, South Korea, China, Japan, Canada, Russia, Singapore, Taiwan, the United Kingdom, Vietnam, Australia, and Philippines.
-
Which are the key categories in the topical absorbable hemostats pipeline market?
The key categories in the topical absorbable hemostats pipeline market are Collagen Based Hemostats, Oxidized Regenerated Cellulose Based Hemostats, Thrombin Based Hemostats, Polysaccharide Based Hemostats, Combination Hemostats – Pads, and Gelatin Based Hemostats.
-
Which are the key regulatory paths in the topical absorbable hemostats pipeline market?
The key regulatory paths in the topical absorbable hemostats pipeline market are 510(k), CE, PMA, ICAC, NMPA, MDITAC, MDL, NIFDS, Ninsho, HSA, BOPA, Shonin, TGA, and UKCA.
-
Which are the key companies in the topical absorbable hemostats pipeline market?
The key companies in the topical absorbable hemostats pipeline market are 3-D Matrix Ltd, Acro Biomedical Co Ltd, Advanced Biotech Products Pvt Ltd, Angiotech Pharmaceuticals Inc, Anika Therapeutics Inc, APRUS Bio-Medical Innovations Pvt Ltd, Arch Therapeutics Inc, Baxter Healthcare Corp, Beth Israel Deaconess Medical Center, Biomedica Management Corp, Biom’Up SAS, and Cellphire Inc.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Wound Care Management reports