Urine Analysis Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Urine Analysis Pipeline Market Report Overview
Urine analysis refers to the analysis of urine to aid in the diagnosis of disease or to detect the presence of a specific substance. The Urine Analysis pipeline market research report provides comprehensive information about the Urine Analysis pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials that are in progress.
| Key Segments | · Urine Analyzers
· Urine Analyzer Reagents |
| Key Territories | · The US
· Europe · South Korea · United Kingdom · Canada |
| Key Regulatory Paths | · 510(k)
· CE-IVD · MDITAC · MDL · Ninsho |
| Leading Companies | · A. Menarini Diagnostics Ltd
· Analyticon Biotechnologies AG · Astrego Diagnostics AB · Bisu Inc · California Pacific Medical Center Research Institute · Clinical Design Technologies Ltd · Eone Diagnomics Genome Center Co Ltd · First Light Diagnostics Inc · Healthy io Ltd · Imperial College London |
| Enquire & Decide | Discover the perfect solution for your business needs. Enquire now and let us help you make an informed decision before making a purchase. |
Urine Analysis Pipeline Market by Segments
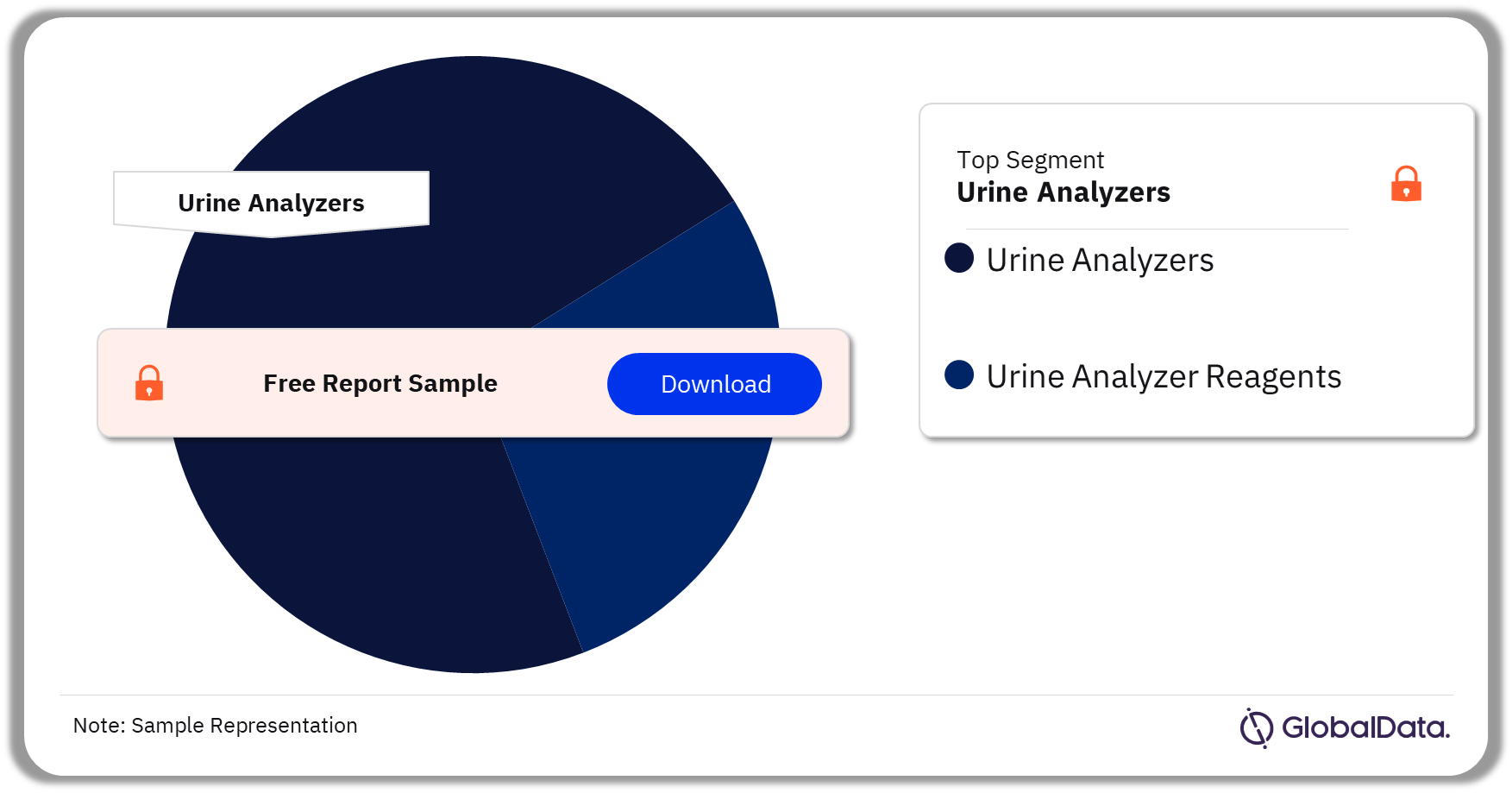
The key segments in the urine analysis pipeline market are urine analyzers and urine analyzer reagents. As of November 2023, urine analyzers has the highest number of pipeline products.
Urine Analysis Pipeline Market Analysis by Segments, 2023 (%)
Buy Full Report for More Segment Insights into the Urine Analysis Pipeline Market
Urine Analysis Pipeline Market Segmentation by Territories
Some of the key territories in the urine analysis market are the US, Europe, South Korea, the UK, and Canada. As of November 2023, the US has the highest number of pipeline products.
Urine Analysis Pipeline Market Analysis by Territories, 2023 (%)
Buy Full Report for More Territory Insights into the Urine Analysis Pipeline Market
Urine Analysis Pipeline Market Segmentation by Regulatory Paths
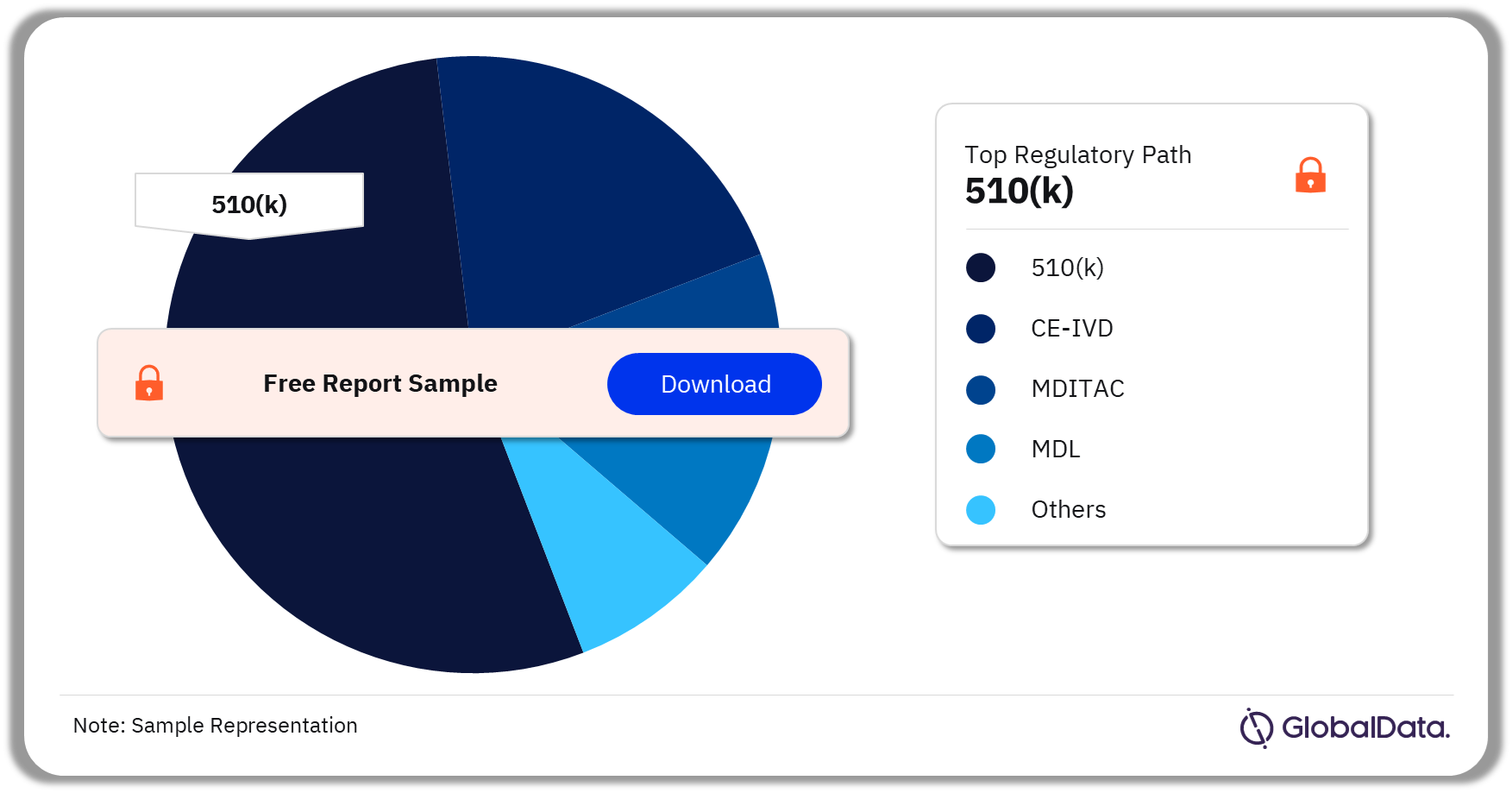
Some of the key regulatory paths in the urine analysis pipeline market are 510(k), CE-IVD, MDITAC, MDL, and Ninsho, among others. As of November 2023, 510(k) is the most followed pathway for pipeline products in the market.
Urine Analysis Pipeline Market Analysis by Regulatory Paths, 2023 (%)
Buy Full Report for More Regulatory Path Insights into the Urine Analysis Pipeline Market
Urine Analysis Pipeline Market – Competitive Landscape
Some of the key companies in the urine analysis pipeline market are A. Menarini Diagnostics Ltd, Analyticon Biotechnologies AG, Astrego Diagnostics AB, Bisu Inc, California Pacific Medical Center Research Institute, Clinical Design Technologies Ltd, Eone Diagnomics Genome Center Co Ltd, First Light Diagnostics Inc, Healthy io Ltd, and Imperial College London.
Analyticon Biotechnologies AG: It is a medical device company that develops, manufactures, and distributes diagnostic systems. The company provides products in the areas of urine diagnostics and hematology. The company’s products are used as diagnostic solutions in physician’s offices, hospitals, and clinical laboratories. It distributes products in Europe, the Middle East, Africa, and Americas through a network of distributors. Analyticon is headquartered in Lichtenfels, Germany.
Sysmex Astrego Diagnostics AB: It is a vitro diagnostic company that provides fully automated Antibiotic Susceptibility Testing (AST) that is quick, sensitive, and accurate for usage in central lab settings and close to patients. The Sysmex Astrego AST system can be utilized for quick susceptibility testing and bacterial infection diagnosis in UTI patients at the Point of Care. Sysmex is headquartered in Uppsala, Sweden.
Urine Analysis Pipeline Market Analysis by Companies, 2023
Buy Full Report for More Company Insights into the Urine Analysis Pipeline Market
Segments Covered in the Report
Urine Analysis Pipeline Market Segments Outlook
- Urine Analyzers
- Urine Analyzer Reagents
Urine Analysis Pipeline Market Territories Outlook
- The US
- Europe
- South Korea
- United Kingdom
- Canada
Urine Analysis Pipeline Market Regulatory Paths Outlook
- 510(k)
- CE-IVD
- MDITAC
- MDL
- Ninsho
Scope
This report provides:
- Extensive coverage of the Urine Analysis under development.
- Details of major pipeline products which include product description, licensing and collaboration details and other developmental activities.
- Reviews of major players involved in the development of Urine Analysis and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Provides key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
This report provides:
- Extensive coverage of the Urine Analysis under development.
- Details of major pipeline products which include product description, licensing and collaboration details and other developmental activities.
- Reviews of major players involved in the development of Urine Analysis and lists all their pipeline projects.
- Coverage of pipeline products based on various stages of development ranging from early development to approved/issued stage.
- Provides key clinical trial data of ongoing clinical trials specific to pipeline products.
- Recent developments in the segment/industry.
Analyticon Biotechnologies AG
Astrego Diagnostics AB
Bisu Inc
California Pacific Medical Center Research Institute
Clinical Design Technologies Ltd
Eone Diagnomics Genome Center Co Ltd
First Light Diagnostics Inc
Healthy io Ltd
Imperial College London
Indian Institute of Technology Delhi
IRIS International Inc
LifeAssays AB
NTBIO Diagnostics Inc
QSTAG Co Ltd
Sebia
Siemens Healthcare Diagnostics Inc
Singapore General Hospital
Stanford University
Teco Diagnostics
University of Kentucky
Withings SA
Table of Contents
Table
Figures
Frequently asked questions
-
Which was the leading segment in the urine analysis pipeline market?
Urine analyzer was the leading segment in the urine analysis pipeline market as of November 2023.
-
Which territory has the highest number of products in the urine analysis pipeline market?
As of November 2023, the US has the highest number of products in the urine analysis pipeline market.
-
Which is the most followed regulatory pathway in the urine analysis pipeline market?
As of November 2023, 510(k) is the most followed pathway for pipeline products in the urine analysis market.
-
Which are the major companies operating in the urine analysis pipeline market?
Some of the key companies in the urine analysis pipeline market are A. Menarini Diagnostics Ltd, Analyticon Biotechnologies AG, Astrego Diagnostics AB, Bisu Inc, California Pacific Medical Center Research Institute, Clinical Design Technologies Ltd, Eone Diagnomics Genome Center Co Ltd, First Light Diagnostics Inc, Healthy io Ltd, and Imperial College London.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Urine Analysis reports