Wound Closure Devices – Pipeline Products by Stage of Development 31
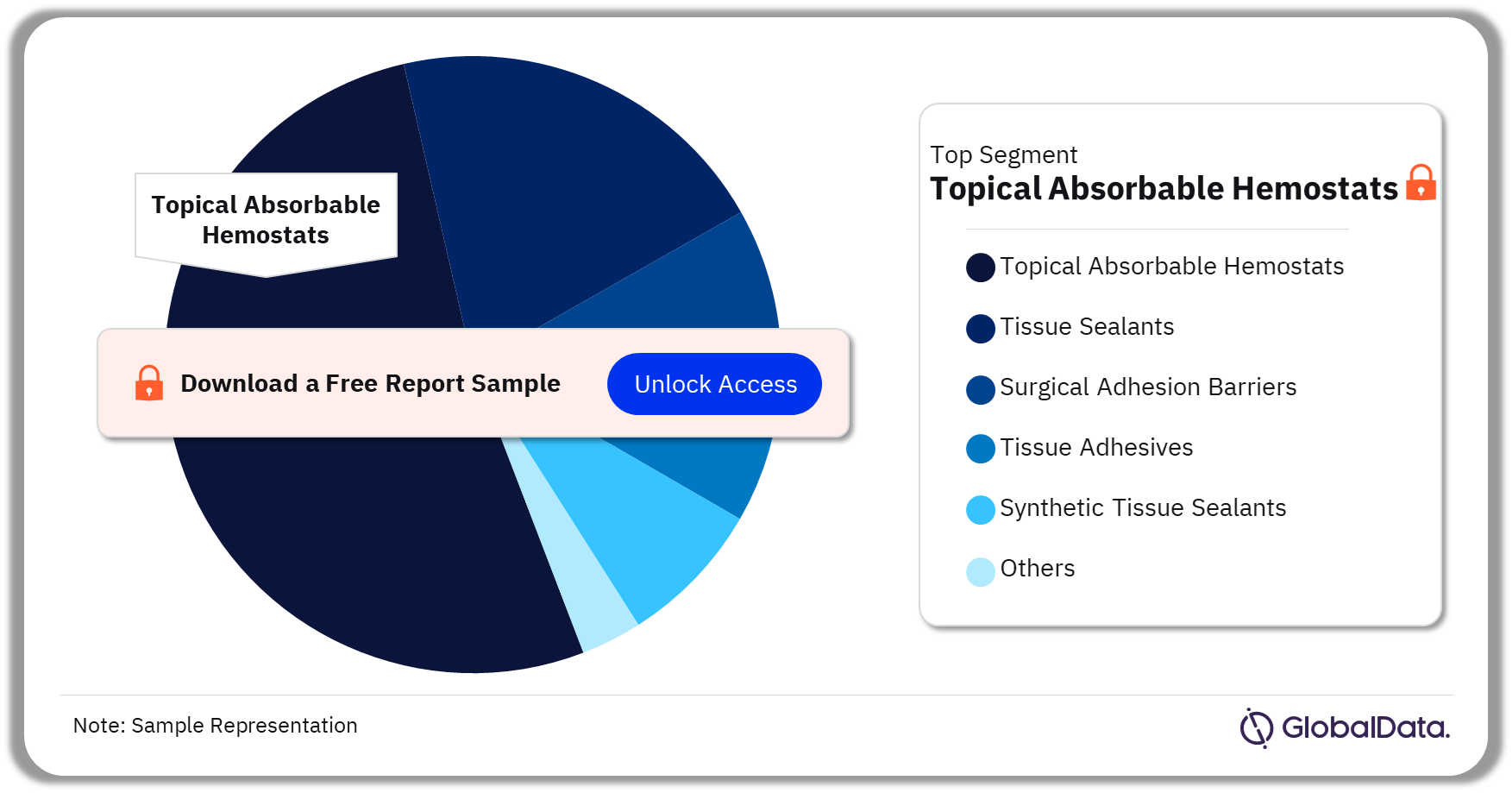
Wound Closure Devices – Pipeline Products by Segment 32
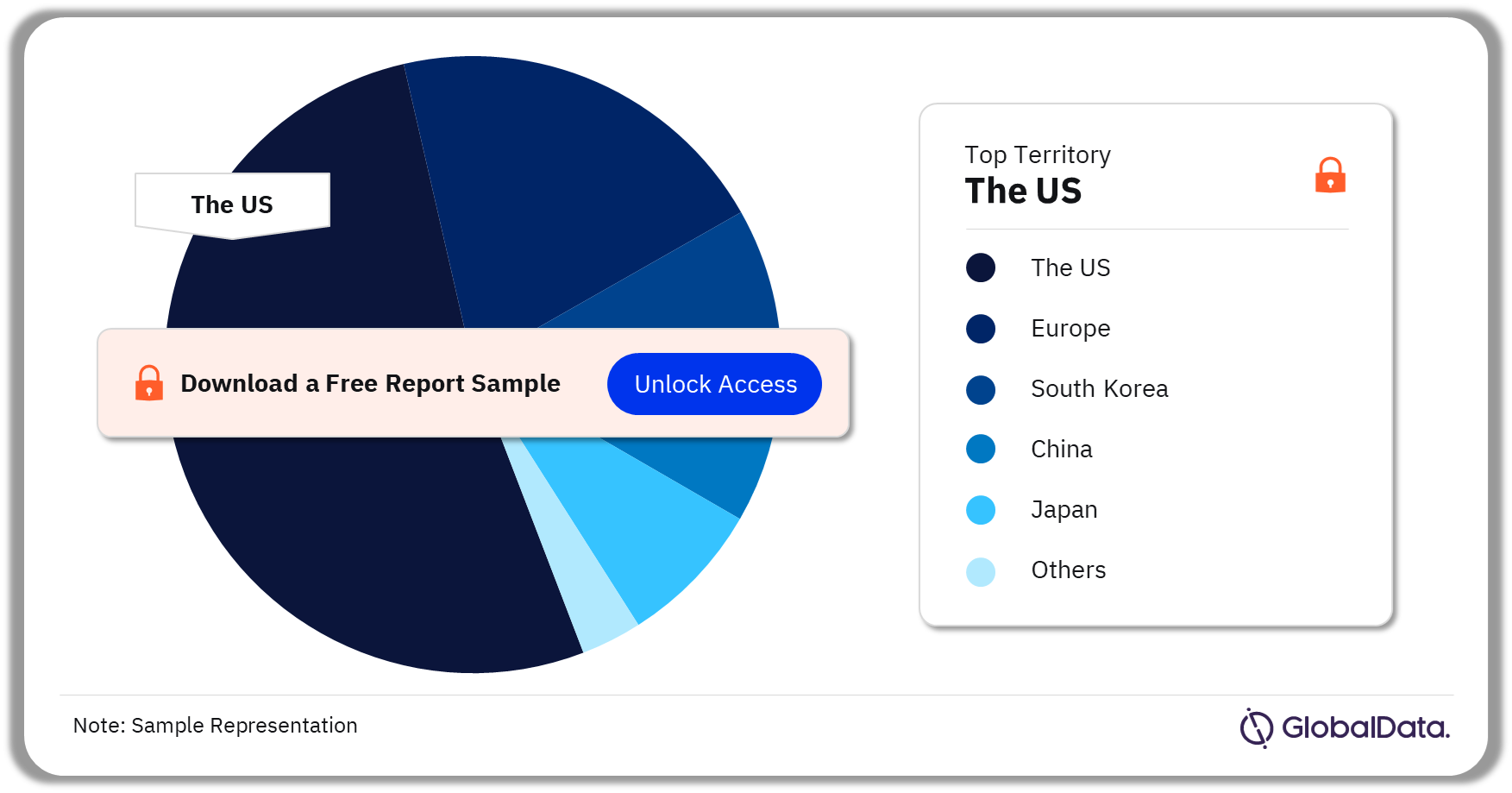
Wound Closure Devices – Pipeline Products by Territory 34
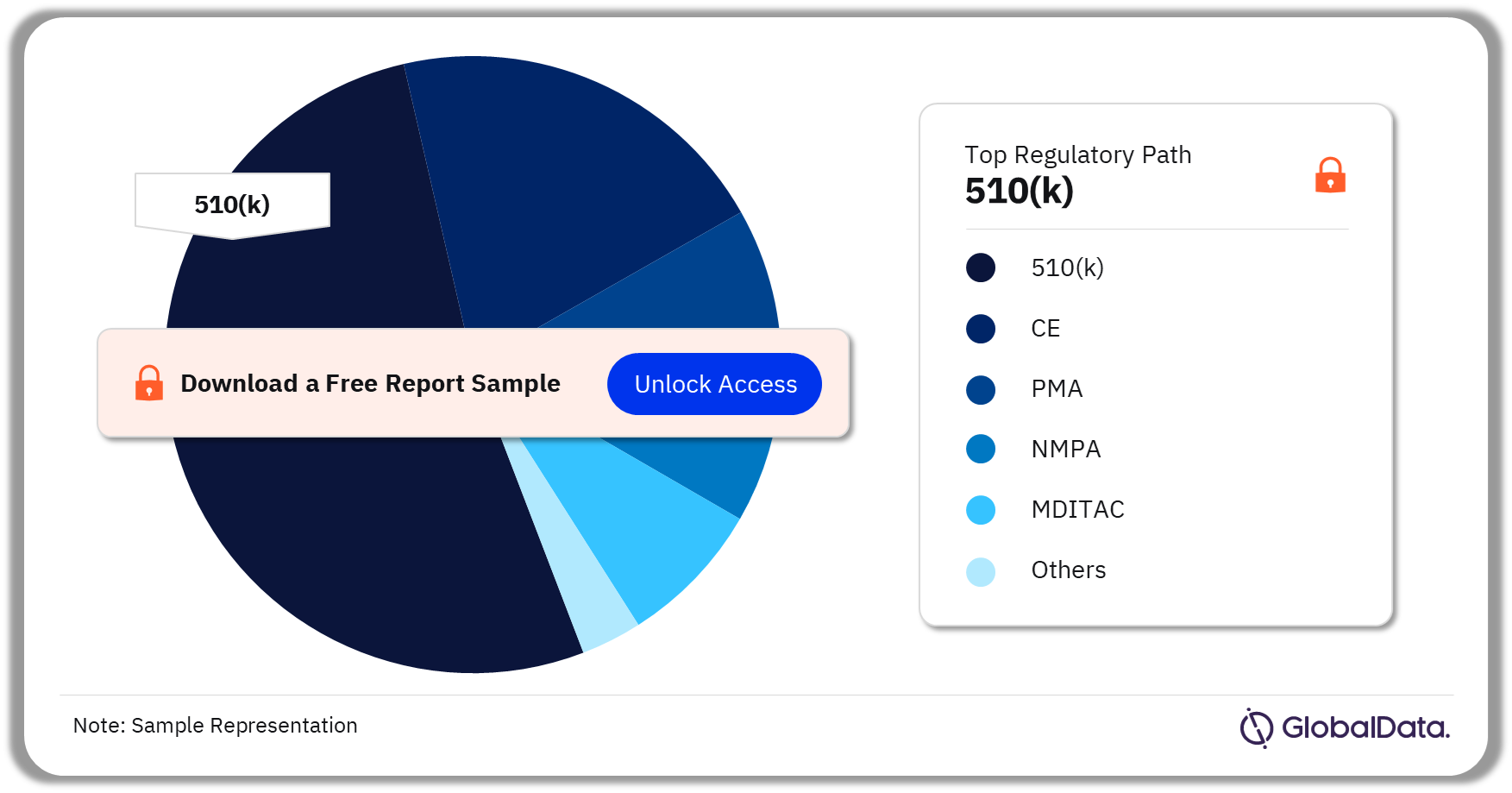
Wound Closure Devices – Pipeline Products by Regulatory Path 36
Wound Closure Devices – Pipeline Products by Estimated Approval Date 37
Wound Closure Devices – Ongoing Clinical Trials 38
Wound Closure Devices Companies – Pipeline Products by Stage of Development 39
Wound Closure Devices – Pipeline Products by Stage of Development 45
3-D Matrix Europe SAS Pipeline Products & Ongoing Clinical Trials Overview 52
NU-MAX – Product Status 52
NU-MAX – Product Description 52
3-D Matrix Europe SAS – Ongoing Clinical Trials Overview 53
NU-MAX – First-in-Human Trial of the Safety and Performance of NU-MAX (IEIK13) as a Hemostatic Agent in Intracranial Neurosurgery 54
3-D Matrix Inc Pipeline Products & Ongoing Clinical Trials Overview 55
PuraStat – Inflammatory Bowel Disease – Product Status 55
PuraStat – Inflammatory Bowel Disease – Product Description 55
3-D Matrix Ltd Pipeline Products & Ongoing Clinical Trials Overview 56
PuraStat – Product Status 56
PuraStat – Product Description 56
TDM-623 – Product Status 57
TDM-623 – Product Description 57
3-D Matrix Ltd – Ongoing Clinical Trials Overview 58
PuraStat – A Multi-center, Single Arm Post-market Clinical Study to Confirm Safety and Performance of Purastat Absorbable Haemostatic Material for the Management of Bleeding after Open Liver Resection 59
PuraStat – A Study to Evaluate the Effectiveness of Purastat in the Prevention of Delayed Bleeding after EMR of Non-ampullary Duodenal Lesions 59
PuraStat – Colorectal Endoscopic Mucosal Resection and Delayed Bleeding: A Prospective Multicentre Randomized Controlled Superiority Trial Comparing PuraStat with Conventional Practice to Reduce the Risk of Delayed Bleeding after Colorectal Endoscopic Mucosal Resection in High-risk Patients 59
PuraStat – Efficacy of PuraStat for the Prevention of Delayed Bleeding after Endoscopic Resection of Colorectal Lesions 60
PuraStat – Endoscopically-delivered Purastat for the Treatment of Haemorrhagic Radiation Proctopathy: A Randomised Feasibility Study 60
PuraStat – Haemostatic Gel Prophylaxis for Post Duodenal Endoscopic Resection Bleeding 60
PuraStat – Prospective, Multicenter Randomized Controlled Trial of the Self-assembling Hemostatic Gel Purastat to Prevent Delayed Bleeding after Endoscopic Resections 61
PuraStat – The Hemostatic Potential of TDM-621 on Ascending Aortic Surgery 61
PuraStat – Treatment of Immediate and Prevention of Delayed Bleeding After Endoscopic Retrograde Cholangiopancreatography Sphincterothomy or Precut with a Novel Self-assembling Peptide Hemostatic Gel. A Single Centre Prospective Observational Nonrandomized Study 61
Abyrx, Inc. Pipeline Products & Ongoing Clinical Trials Overview 62
Soft Tissue Hemostat – Product Status 62
Soft Tissue Hemostat – Product Description 62
ACell Inc Pipeline Products & Ongoing Clinical Trials Overview 63
MicroMatrix – Product Status 63
MicroMatrix – Product Description 63
Acro Biomedical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 64
ACROHCM – Product Status 64
ACROHCM – Product Description 64
Actamax Surgical Materials LLC Pipeline Products & Ongoing Clinical Trials Overview 65
Actamax Adhesion Barrier – Product Status 65
Actamax Adhesion Barrier – Product Description 65
Actamax Surgical Materials LLC – Ongoing Clinical Trials Overview 66
Actamax Adhesion Barrier – A Randomized, Controlled, Multi-center Study to Assess the Safety and Efficacy of Actamax Adhesion Barrier in Women undergoing Laparoscopic Abdominopelvic Surgery with a Myomectomy Followed by Second Look Laparoscopy (SLL) 67
Adhesys Medical GmbH Pipeline Products & Ongoing Clinical Trials Overview 68
Vivo – Product Status 68
Vivo – Product Description 68
Advanced Biotech Products Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 69
Hemocoll – Product Status 69
Hemocoll – Product Description 69
Advanced Medical Solutions Group plc Pipeline Products & Ongoing Clinical Trials Overview 70
Large Wounds Closure Device – Product Status 70
Large Wounds Closure Device – Product Description 70
LiquiBand – Product Status 71
LiquiBand – Product Description 71
Next Generation Internal Fixation And Sealant Device – Product Status 72
Next Generation Internal Fixation And Sealant Device – Product Description 72
Advanced Medical Solutions Group plc – Ongoing Clinical Trials Overview 73
LiquiBand – A Prospective Randomized Controlled Trial Comparing Cyanoacrylate Skin Adhesive with Staple for Surgical Incision Closure after Laparoscopic / Robotic Bowel Resection 74
AFYX Therapeutics A/S Pipeline Products & Ongoing Clinical Trials Overview 75
Rivelin Plain Patch – Product Status 75
Rivelin Plain Patch – Product Description 75
Alafair Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 76
VersaWrap Adhesion Barrier – Product Status 76
VersaWrap Adhesion Barrier – Product Description 76
Albert Ludwigs University of Freiburg Pipeline Products & Ongoing Clinical Trials Overview 77
Biohybrid Membrane – Product Status 77
Biohybrid Membrane – Product Description 77
Aleo BME Inc Pipeline Products & Ongoing Clinical Trials Overview 78
BP Glue – Product Status 78
BP Glue – Product Description 78
Dural Sealant – Product Status 79
Dural Sealant – Product Description 79
Surgical Sealant – Product Status 79
Surgical Sealant – Product Description 79
Topical Adhesive – Product Status 80
Topical Adhesive – Product Description 80
Alumend LLC Pipeline Products & Ongoing Clinical Trials Overview 81
Tissue-Bonding Adhesive – Product Status 81
Tissue-Bonding Adhesive – Product Description 81
Angiotech Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 82
Hemaseel HMN Fibrin Sealant – Product Status 82
Hemaseel HMN Fibrin Sealant – Product Description 82
Hemaseel Thrombin Haemostatic Agent – Product Status 83
Hemaseel Thrombin Haemostatic Agent – Product Description 83
Anika Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 84
Hemostatic Patch – Product Status 84
Hemostatic Patch – Product Description 84
HYAFF Gel – Product Status 85
HYAFF Gel – Product Description 85
APRUS Bio-Medical Innovations Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 86
Deep Wound Hemostat – Product Status 86
Deep Wound Hemostat – Product Description 86
ARC Medical Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 87
Discrete – Product Status 87
Discrete – Product Description 87
Arch Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 88
AC5 – Adhesion Prevention – Product Status 88
AC5 – Adhesion Prevention – Product Description 89
AC5 – Burns – Product Status 89
AC5 – Burns – Product Description 89
AC5 – Diabetic Ulcers – Product Status 90
AC5 – Diabetic Ulcers – Product Description 90
AC5 – Pressure Ulcers – Product Status 90
AC5 – Pressure Ulcers – Product Description 91
AC5 – V – Product Status 91
AC5 – V – Product Description 91
AC5 Surgery – Fluid Barrier – Product Status 92
AC5 Surgery – Fluid Barrier – Product Description 92
AC5 Surgery – Laparascopic Procedures – Product Status 92
AC5 Surgery – Laparascopic Procedures – Product Description 93
AC5 Surgery – Open Procedures – Product Status 93
AC5 Surgery – Open Procedures – Product Description 93
AC5 Surgery – Sealant – Product Status 94
AC5 Surgery – Sealant – Product Description 94
AC5 Surgical Hemostatic Device – Product Status 94
AC5 Surgical Hemostatic Device – Product Description 95
AC5-G – Product Status 95
AC5-G – Product Description 95
Arch Therapeutics Inc – Ongoing Clinical Trials Overview 96
AC5 – V – Study to Differentiate the Key Benefits of Advanced Wound System (A Multi-Site Clinical Study) 97
Arizona State University Pipeline Products & Ongoing Clinical Trials Overview 98
Laser-Activated Nanosealant – Product Status 98
Laser-Activated Nanosealant – Product Description 98
Artivion Inc Pipeline Products & Ongoing Clinical Trials Overview 99
Bioglue Surgical Adhesive – Product Status 99
Bioglue Surgical Adhesive – Product Description 100
Artivion Inc – Ongoing Clinical Trials Overview 101
Bioglue Surgical Adhesive – A Prospective, Multicenter Study of BioGlue in the Treatment of Chinese Patients with Acute Type A Aortic Dissections 102
Bioglue Surgical Adhesive – Multi-center, Randomized, Controlled Clinical Trial of BioGlue Surgical Adhesive as an Adjunct for Structural Repair and Hemostasis in Chinese Patients with Acute Type A Aortic Dissections 102
Aura Medsystems, Inc. Pipeline Products & Ongoing Clinical Trials Overview 103
LaSTR – Skin Closure Device – Product Status 103
LaSTR – Skin Closure Device – Product Description 103
BaroStitch Pipeline Products & Ongoing Clinical Trials Overview 104
BaroStitch – Product Status 104
BaroStitch – Product Description 104
Baxter International Inc Pipeline Products & Ongoing Clinical Trials Overview 105
ACCUREACH – Product Status 105
ACCUREACH – Product Description 105
Floseal + Recothrom 10mL – Product Status 106
Floseal + Recothrom 10mL – Product Description 106
SEPRALAP – Product Status 106
SEPRALAP – Product Description 107
Tisseel Dura – Product Status 107
Tisseel Dura – Product Description 107
Bayer AG Pipeline Products & Ongoing Clinical Trials Overview 108
Polyurethane Adhesive System – Product Status 108
Polyurethane Adhesive System – Product Description 108
Bergen Medical Products Inc Pipeline Products & Ongoing Clinical Trials Overview 109
ACTACLOSE – Product Status 109
ACTACLOSE – Product Description 109
Beth Israel Deaconess Medical Center Pipeline Products & Ongoing Clinical Trials Overview 110
Portable Wound Hemostasis System – Product Status 110
Portable Wound Hemostasis System – Product Description 110
BioActive Polymers in Lund AB Pipeline Products & Ongoing Clinical Trials Overview 111
BioBarrier – Product Status 111
BioBarrier – Product Description 111
BioDevek Inc Pipeline Products & Ongoing Clinical Trials Overview 112
Adhesive Sealant – Product Status 112
Adhesive Sealant – Product Description 112
Biomedica Management Corp Pipeline Products & Ongoing Clinical Trials Overview 113
ClotBlock – Product Status 113
ClotBlock – Product Description 113
ClotFoam – Product Status 114
ClotFoam – Product Description 114
ClotGel – Product Status 114
ClotGel – Product Description 115
ClotSpray – Product Status 115
ClotSpray – Product Description 115
Biom’Up SAS Pipeline Products & Ongoing Clinical Trials Overview 116
HEMOSNOW – Product Status 116
HEMOSNOW – Product Description 116
BNC Korea Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 117
BNC-RB01 – Product Status 117
BNC-RB01 – Product Description 117
BNC-RH01 – Product Status 118
BNC-RH01 – Product Description 118
Borayer Biotechnology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 119
Biofluid Hemostatic Membrane – Product Status 119
Biofluid Hemostatic Membrane – Product Description 119
Boston University Pipeline Products & Ongoing Clinical Trials Overview 120
Hydrogel-Based Aerosolized Sealant Dressing – Product Status 120
Hydrogel-Based Aerosolized Sealant Dressing – Product Description 120
Calmare Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 121
Wound Sealant – Product Status 121
Wound Sealant – Product Description 122
CardioCommand Inc Pipeline Products & Ongoing Clinical Trials Overview 123
Tournicath Device – Product Status 123
Tournicath Device – Product Description 123
Cellphire Inc Pipeline Products & Ongoing Clinical Trials Overview 124
Thromboderm – Product Status 124
Thromboderm – Product Description 124
Thrombosomes – Product Status 125
Thrombosomes – Product Description 125
Ceramicos Para Aplicacoes Medicas SA Pipeline Products & Ongoing Clinical Trials Overview 126
HemoKi – Product Status 126
HemoKi – Product Description 126
Clemson University Pipeline Products & Ongoing Clinical Trials Overview 127
Surgical Adhesive Hydrogel – Product Status 127
Surgical Adhesive Hydrogel – Product Description 127
Cohera Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 128
Sylys Surgical Sealant – Drug Delivery – Product Status 128
Sylys Surgical Sealant – Drug Delivery – Product Description 128
Sylys Surgical Sealant – Hernia Mesh Fixation – Product Status 129
Sylys Surgical Sealant – Hernia Mesh Fixation – Product Description 129
Sylys Surgical Sealant – Orthopedic – Product Status 129
Sylys Surgical Sealant – Orthopedic – Product Description 130
Columbia University Pipeline Products & Ongoing Clinical Trials Overview 131
Hemostatic Wound Dressing – Product Status 131
Hemostatic Wound Dressing – Product Description 131
Covalent Medical, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 132
Covabond – Product Status 132
Covabond – Product Description 132
CovaSTAT – Product Status 133
CovaSTAT – Product Description 133
Covalon Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 134
ColActive THP – Product Status 134
ColActive THP – Product Description 134
CovaStat BioActive Absorbable Hemostatic Sponge – Product Status 135
CovaStat BioActive Absorbable Hemostatic Sponge – Product Description 135
Covidien Ltd Pipeline Products & Ongoing Clinical Trials Overview 136
Soft Tissue Repair Device – Product Status 136
Soft Tissue Repair Device – Product Description 136
CR Bard Inc Pipeline Products & Ongoing Clinical Trials Overview 137
Absorbable Hemostat – Product Status 137
Absorbable Hemostat – Product Description 137
New Hemostat 1 – Product Status 138
New Hemostat 1 – Product Description 138
New Hemostat 2 – Product Status 138
New Hemostat 2 – Product Description 138
Progel Dural Sealant – Product Status 139
Progel Dural Sealant – Product Description 139
Progel Emerald – Product Status 139
Progel Emerald – Product Description 139
ProGEL-VS – Product Status 140
ProGEL-VS – Product Description 140
Tissue Adhesive – Product Status 140
Tissue Adhesive – Product Description 141
Tridyne Peripheral – Product Status 141
Tridyne Peripheral – Product Description 141
Cresilon Inc Pipeline Products & Ongoing Clinical Trials Overview 142
TRAUMAGEL – Product Status 142
TRAUMAGEL – Product Description 142
Department of Biomedical Engineering Columbia University Pipeline Products & Ongoing Clinical Trials Overview 143
Biomimetic Lung Sealant – Product Status 143
Biomimetic Lung Sealant – Product Description 143
DSM Biomedical BV Pipeline Products & Ongoing Clinical Trials Overview 144
Dural Membrane Sealant – Product Status 144
Dural Membrane Sealant – Product Description 144
Duke University Pipeline Products & Ongoing Clinical Trials Overview 145
Intrauterine Adhesion Barrier – Product Status 145
Intrauterine Adhesion Barrier – Product Description 145
Eidgenossische Materialprufungs- und Forschungsanstalt Pipeline Products & Ongoing Clinical Trials Overview 146
Polymer Patch – Product Status 146
Polymer Patch – Product Description 146
Elastagen Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 147
MeTro – Surgical Glue – Product Status 147
MeTro – Surgical Glue – Product Description 147
Emory University Pipeline Products & Ongoing Clinical Trials Overview 148
Steroidal Hemostatic Surgical Adhesive – Product Status 148
Steroidal Hemostatic Surgical Adhesive – Product Description 148
Endomedix Inc Pipeline Products & Ongoing Clinical Trials Overview 149
Surgical Hemostat – Intracranial Surgery – Product Status 149
Surgical Hemostat – Intracranial Surgery – Product Description 149
Topical Hemostat – Product Status 150
Topical Hemostat – Product Description 150
Entegrion Inc Pipeline Products & Ongoing Clinical Trials Overview 151
Stasix – Product Status 151
Stasix – Product Description 151
Ethicon Endo-Surgery Inc Pipeline Products & Ongoing Clinical Trials Overview 152
Bioseal Next Gen Device – Product Status 152
Bioseal Next Gen Device – Product Description 152
Ethicon US LLC Pipeline Products & Ongoing Clinical Trials Overview 153
Next Gen Hemostat Kit – Product Status 153
Next Gen Hemostat Kit – Product Description 153
PRINEO Next Generation – Product Status 154
PRINEO Next Generation – Product Description 154
SURGIFLO – Sterile Field Preparation – Product Status 154
SURGIFLO – Sterile Field Preparation – Product Description 155
Ethicon US LLC – Ongoing Clinical Trials Overview 156
PRINEO Next Generation – The Effects of Post-Operative Interventions on Surgical Site Occurrences in Abdominal Wall Reconstructions: A Randomized Controlled Trial 157
ETX Pharma Inc Pipeline Products & Ongoing Clinical Trials Overview 158
ETX-302 – Product Status 158
ETX-302 – Product Description 158
Fistula Solution Corp Pipeline Products & Ongoing Clinical Trials Overview 159
Limpet – Product Status 159
Limpet – Product Description 159
FzioMed Inc Pipeline Products & Ongoing Clinical Trials Overview 160
Oxiplex/AP – Product Status 160
Oxiplex/AP – Product Description 160
Oxiplex/IU – Product Status 161
Oxiplex/IU – Product Description 161
Gamma Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview 162
GammaSeal – Product Status 162
GammaSeal – Product Description 162
GammaTF – Product Status 163
GammaTF – Product Description 163
GATT Technologies BV Pipeline Products & Ongoing Clinical Trials Overview 164
GATT-Patch – Product Status 164
GATT-Patch – Product Description 164
GATT-Powder – Product Status 165
GATT-Powder – Product Description 165
GATT-Spray – Product Status 165
GATT-Spray – Product Description 166
GATT-Tape – Product Status 166
GATT-Tape – Product Description 166
GATT Technologies BV – Ongoing Clinical Trials Overview 167
GATT-Patch – A Prospective, Multicenter, Randomized Clinical Investigation Evaluating GATT-Patch for Hemostasis During Minimally Invasive Liver and Gallbladder Surgery 168
Genzyme Corp Pipeline Products & Ongoing Clinical Trials Overview 169
Sepraspray Adhesion Barrier – Product Status 169
Sepraspray Adhesion Barrier – Product Description 169
Haemostatix Ltd Pipeline Products & Ongoing Clinical Trials Overview 170
Haemoplax – Product Status 170
Haemoplax – Product Description 170
PeproStat – Product Status 171
PeproStat – Product Description 171
Hangzhou Kangji Medical Instrument Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 172
Automatic-Loading Ligation Clips & Applier – Product Status 172
Automatic-Loading Ligation Clips & Applier – Product Description 172
Harvard University Pipeline Products & Ongoing Clinical Trials Overview 173
Tough Adhesive – Product Status 173
Tough Adhesive – Product Description 173
Hemostasis LLC Pipeline Products & Ongoing Clinical Trials Overview 174
ACCEL Absorbable Hemostat – Product Status 174
ACCEL Absorbable Hemostat – Product Description 174
Advanced Tissue Sealant – Product Status 175
Advanced Tissue Sealant – Product Description 175
ENT Hemostat – Product Status 175
ENT Hemostat – Product Description 175
Hemostatic Gel – Product Status 176
Hemostatic Gel – Product Description 176
Sealant Applicator – Product Status 176
Sealant Applicator – Product Description 176
Hemostasis LLC – Ongoing Clinical Trials Overview 177
ACCEL Absorbable Hemostat – ACCEL Absorbable Hemostat Powder Clinical Trial 178
HLL Lifecare Ltd Pipeline Products & Ongoing Clinical Trials Overview 179
Hinstat Ext – Product Status 179
Hinstat Ext – Product Description 179
HydroGlaze BV Pipeline Products & Ongoing Clinical Trials Overview 180
HydroGlaze – Product Status 180
HydroGlaze – Product Description 180
HyperBranch Medical Technology Inc Pipeline Products & Ongoing Clinical Trials Overview 181
Adherus Vascular Sealant – Product Status 181
Adherus Vascular Sealant – Product Description 181
Tissue Plane Sealant – Product Status 182
Tissue Plane Sealant – Product Description 182
Ichor Sciences LLC Pipeline Products & Ongoing Clinical Trials Overview 183
Statbond ENT – Product Status 183
Statbond ENT – Product Description 183
Innocoll Technologies Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 184
CM004 – Product Status 184
CM004 – Product Description 184
Innotherapy Inc Pipeline Products & Ongoing Clinical Trials Overview 185
GellySil – Product Status 185
GellySil – Product Description 185
Surgical Sealant Film – Product Status 186
Surgical Sealant Film – Product Description 186
Institute of Molecular Biology of Parana Pipeline Products & Ongoing Clinical Trials Overview 187
Recombinant Fibrin Glue – Product Status 187
Recombinant Fibrin Glue – Product Description 187
InVivo Therapeutics Corp Pipeline Products & Ongoing Clinical Trials Overview 188
InVivoSeal – Product Status 188
InVivoSeal – Product Description 188
IonMed Ltd. Pipeline Products & Ongoing Clinical Trials Overview 189
BioWeld1 System – Acne – Product Status 189
BioWeld1 System – Acne – Product Description 189
BioWeld1 System – Burns And Chronic Wounds – Product Status 190
BioWeld1 System – Burns And Chronic Wounds – Product Description 190
BioWeld1 System – Skin Graft Fixation – Product Status 190
BioWeld1 System – Skin Graft Fixation – Product Description 191
Jiangxi Boenruier Biotechnology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 192
Fluid Hemostatic Agent – Product Status 192
Fluid Hemostatic Agent – Product Description 192
Jinwoo Bio Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 193
HA Fabric Sheet Adhesive Barrier – Product Status 193
HA Fabric Sheet Adhesive Barrier – Product Description 194
HA Film Adhesive Barrier – Product Status 194
HA Film Adhesive Barrier – Product Description 194
Wool Adhesive Barrier – Product Status 195
Wool Adhesive Barrier – Product Description 195
Johns Hopkins University Pipeline Products & Ongoing Clinical Trials Overview 196
Chondroitin Sulfate-based Adhesive – Product Status 196
Chondroitin Sulfate-based Adhesive – Product Description 196
Keratin Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 197
Keratin-Based Hemostat – Product Status 197
Keratin-Based Hemostat – Product Description 197
Kollodis BioSciences Inc Pipeline Products & Ongoing Clinical Trials Overview 198
Cartilage Adhesive – Product Status 198
Cartilage Adhesive – Product Description 198
Hemostat Sealant – Product Status 198
Hemostat Sealant – Product Description 199
Kuros Biosciences AG Pipeline Products & Ongoing Clinical Trials Overview 200
KUR – 211 – Product Status 200
KUR – 211 – Product Description 200
KUR – 212 – Product Status 201
KUR – 212 – Product Description 201
Kytogenics Pharmaceuticals Inc Pipeline Products & Ongoing Clinical Trials Overview 202
Adhes-X – Product Status 202
Adhes-X – Product Description 202
Lap IQ Inc Pipeline Products & Ongoing Clinical Trials Overview 203
FAStlink – Product Status 203
FAStlink – Product Description 203
Laser Tissue Welding, Inc. Pipeline Products & Ongoing Clinical Trials Overview 204
Laser Tissue Welding Device – Product Status 204
Laser Tissue Welding Device – Product Description 204
Latch Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 205
Relialoc Skin Closure System – Product Status 205
Relialoc Skin Closure System – Product Description 205
Leader Biomedical Europe BV Pipeline Products & Ongoing Clinical Trials Overview 206
Collagen-Based Haemostatic Device – Product Status 206
Collagen-Based Haemostatic Device – Product Description 206
Rebasol – Product Status 207
Rebasol – Product Description 207
Lenvitz Medical Solutions Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 208
Auto Clip Applicator – Product Status 208
Auto Clip Applicator – Product Description 208
Life Care Medical Devices Ltd Pipeline Products & Ongoing Clinical Trials Overview 209
LCMD-004 – Product Status 209
LCMD-004 – Product Description 209
Luna Innovations Inc Pipeline Products & Ongoing Clinical Trials Overview 210
Sutureless Absorbable Antiadhesion Film – Product Status 210
Sutureless Absorbable Antiadhesion Film – Product Description 210
TissuCoat – Product Status 211
TissuCoat – Product Description 211
Magen OrthoMed Ltd Pipeline Products & Ongoing Clinical Trials Overview 212
NerveShield – Product Status 212
NerveShield – Product Description 212
TendonShield – Product Status 213
TendonShield – Product Description 213
Massachusetts Institute of Technology Pipeline Products & Ongoing Clinical Trials Overview 214
Double-Sided Tape – Product Status 214
Double-Sided Tape – Product Description 214
Surgical Tape – Product Status 215
Surgical Tape – Product Description 215
Maxigen Biotech Inc Pipeline Products & Ongoing Clinical Trials Overview 216
MaxiGel – Product Status 216
MaxiGel – Product Description 216
Medcura Inc Pipeline Products & Ongoing Clinical Trials Overview 217
Hemogrip Foam – Product Status 217
Hemogrip Foam – Product Description 217
LifeDust – Product Status 218
LifeDust – Product Description 218
LifeFoam – Product Status 218
LifeFoam – Product Description 219
LifeGel – Product Status 219
LifeGel – Product Description 219
Medical Plasmas SL Pipeline Products & Ongoing Clinical Trials Overview 220
PlasmAction – Product Status 220
PlasmAction – Product Description 220
Medical Plasmas SL – Ongoing Clinical Trials Overview 221
PlasmAction – Air Cold Atmospheric Pressure Plasma Treatment for Acceleration of Venous Ulcer Healing 222
Medisse BV (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 223
FlexiSurge Adhesive Barrier – Product Status 223
FlexiSurge Adhesive Barrier – Product Description 223
PTMC Adhesive – Product Status 224
PTMC Adhesive – Product Description 224
PTMC Sealant – Product Status 224
PTMC Sealant – Product Description 225
Meril Life Sciences Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview 226
PeriTi – Product Status 226
PeriTi – Product Description 226
MLM Biologics Inc Pipeline Products & Ongoing Clinical Trials Overview 227
Adhesion Barrier – Product Status 227
Adhesion Barrier – Product Description 227
Biologic Barrier Membrane – Superficial Wounds – Product Status 228
Biologic Barrier Membrane – Superficial Wounds – Product Description 228
N8 Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 229
Tissue Glue & Microbial Sealent – Product Status 229
Tissue Glue & Microbial Sealent – Product Description 229
Wound Closure Adhesive – Product Status 230
Wound Closure Adhesive – Product Description 230
Nanyang Technological University Pipeline Products & Ongoing Clinical Trials Overview 231
CaproGlu – Product Status 231
CaproGlu – Product Description 231
National University of Singapore Pipeline Products & Ongoing Clinical Trials Overview 232
Colon Patch – Product Status 232
Colon Patch – Product Description 232
Northwestern University Pipeline Products & Ongoing Clinical Trials Overview 233
Medical Adhesive – Product Status 233
Medical Adhesive – Product Description 233
Nurami Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 234
ArtiFix – Product Status 234
ArtiFix – Product Description 234
OptMed, Inc. Pipeline Products & Ongoing Clinical Trials Overview 235
Biosurgical Adhesive – Chronic Wounds – Product Status 235
Biosurgical Adhesive – Chronic Wounds – Product Description 235
Biosurgical Adhesive – Internal Use – Product Status 236
Biosurgical Adhesive – Internal Use – Product Description 236
Biosurgical Adhesive – Skin Tears – Product Status 236
Biosurgical Adhesive – Skin Tears – Product Description 237
Paul Scherrer Institute Pipeline Products & Ongoing Clinical Trials Overview 238
Omnibond Topical Skin Adhesive – Product Status 238
Omnibond Topical Skin Adhesive – Product Description 238
Paul Scherrer Institute – Ongoing Clinical Trials Overview 239
Omnibond Topical Skin Adhesive – A Prospective, Randomized, Single-blinded Study to Assess the Incidence of Wound Complications Following Total Knee and Hip Arthroplasty in Patients Treated with Two Different Types of Topical Skin Adhesive 240
PetVivo Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 241
Vasculitus Wound Closure Device – Product Status 241
Vasculitus Wound Closure Device – Product Description 241
Pharming Group NV Pipeline Products & Ongoing Clinical Trials Overview 242
Recombinant Fibrin Tissue Sealant – Product Status 242
Recombinant Fibrin Tissue Sealant – Product Description 242
Polyganics BV Pipeline Products & Ongoing Clinical Trials Overview 243
3D Printed Scaffold – Product Status 243
3D Printed Scaffold – Product Description 243
ACTISEAL – Product Status 244
ACTISEAL – Product Description 244
ACTISEAL – Adjacent Indications – Product Status 244
ACTISEAL – Adjacent Indications – Product Description 245
LIQOSEAL – Dura Sealing Patch – Product Status 245
LIQOSEAL – Dura Sealing Patch – Product Description 245
LIQOSEAL – Spine Application – Product Status 246
LIQOSEAL – Spine Application – Product Description 246
Polyganics BV – Ongoing Clinical Trials Overview 247
LIQOSEAL – Dura Sealing Patch – Randomized, Two-arm, Multicenter Study to Evaluate the Safety and Efficacy of Dura Sealant Patch in Reducing CSF Leakage Following Elective Cranial Surgery 248
PolyNovo Biomaterials Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview 249
Anti-Adhesion Film – Product Status 249
Anti-Adhesion Film – Product Description 249
Progress and Health Foundation Pipeline Products & Ongoing Clinical Trials Overview 250
Wound Closure Kit – Product Status 250
Wound Closure Kit – Product Description 250
Protege Biomedical LLC Pipeline Products & Ongoing Clinical Trials Overview 251
Ionic Hemostatic Mineral Bandage – Emergency Trauma – Product Status 251
Ionic Hemostatic Mineral Bandage – Emergency Trauma – Product Description 251
Protein Polymer Technologies Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 252
Surgical Tissue Sealant – Product Status 252
Surgical Tissue Sealant – Product Description 252
Purdue University Pipeline Products & Ongoing Clinical Trials Overview 253
Surgical Adhesive – Product Status 253
Surgical Adhesive – Product Description 253
Surgical Sealant – Product Status 254
Surgical Sealant – Product Description 254
Radiation Monitoring Devices Inc Pipeline Products & Ongoing Clinical Trials Overview 255
Hemostatic Patch – Traumatic Injury – Product Status 255
Hemostatic Patch – Traumatic Injury – Product Description 255
Regenics AS Pipeline Products & Ongoing Clinical Trials Overview 256
Collex – Product Status 256
Collex – Product Description 256
Vernex – Product Status 257
Vernex – Product Description 257
Resorba GmbH Pipeline Products & Ongoing Clinical Trials Overview 258
Antibiotic Hemostat – Product Status 258
Antibiotic Hemostat – Product Description 258
RESORBA – Cardiac Surgery – Product Status 259
RESORBA – Cardiac Surgery – Product Description 259
Rice University Pipeline Products & Ongoing Clinical Trials Overview 260
KOD Hydrogel Hemostat – Product Status 260
KOD Hydrogel Hemostat – Product Description 260
Rutgers The State University of New Jersey Pipeline Products & Ongoing Clinical Trials Overview 261
Biodegradable Film Implant – Product Status 261
Biodegradable Film Implant – Product Description 261
Samyang Biopharmaceuticals Corp Pipeline Products & Ongoing Clinical Trials Overview 262
Surgical Sealant – Product Status 262
Surgical Sealant – Product Description 262
Sanofi Biosurgery Inc Pipeline Products & Ongoing Clinical Trials Overview 263
LeSeal – Product Status 263
LeSeal – Product Description 263
Lumagel – Product Status 264
Lumagel – Product Description 264
Sea Run Holdings Inc Pipeline Products & Ongoing Clinical Trials Overview 265
SEA-STAT – Product Status 265
SEA-STAT – Product Description 265
SEA-STAT CNS – Product Status 266
SEA-STAT CNS – Product Description 266
Sealantis Ltd. Pipeline Products & Ongoing Clinical Trials Overview 267
Seal-G – Product Status 267
Seal-G – Product Description 267
Seal-G MIST – Product Status 268
Seal-G MIST – Product Description 268
Sealantis Tissue Adhesive – Product Status 268
Sealantis Tissue Adhesive – Product Description 269
Sealantis Ltd. – Ongoing Clinical Trials Overview 270
Seal-G – Seal-G and Seal-G MIST Study for Reinforcement and Protection of Colonic Anastomoses in Subjects Undergoing Colonic Resection 271
Seal-G MIST – Seal-G and Seal-G MIST Study for Reinforcement and Protection of Colonic Anastomoses in Subjects Undergoing Colonic Resection 272
Seikagaku Corp Pipeline Products & Ongoing Clinical Trials Overview 273
SI-449 Adhesion Barrier System – Product Status 273
SI-449 Adhesion Barrier System – Product Description 273
Seikagaku Corp – Ongoing Clinical Trials Overview 274
SI-449 Adhesion Barrier System – A Randomized, Controlled, Pivotal Study of SI-449 Adhesion Barrier in Patients with Temporary Ileostomy 275
Selio Medical Pipeline Products & Ongoing Clinical Trials Overview 276
Selio Sealant System – Product Status 276
Glossary
![]()