Interventional Neuroradiology – Pipeline Products by Stage of Development 31
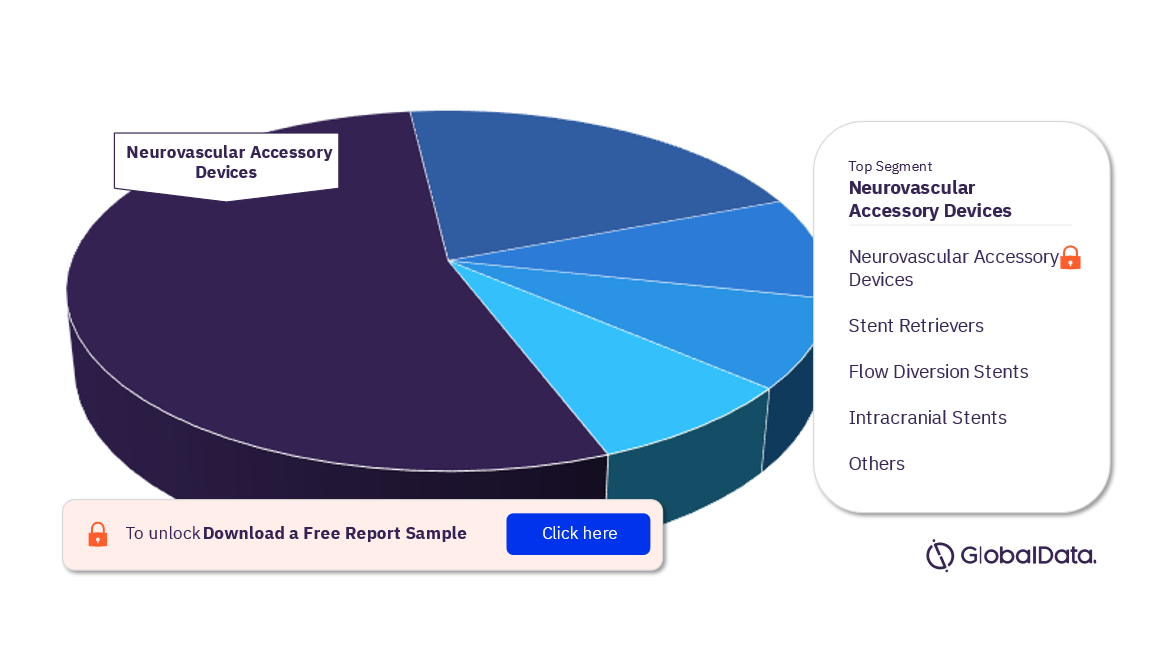
Interventional Neuroradiology – Pipeline Products by Segment 32
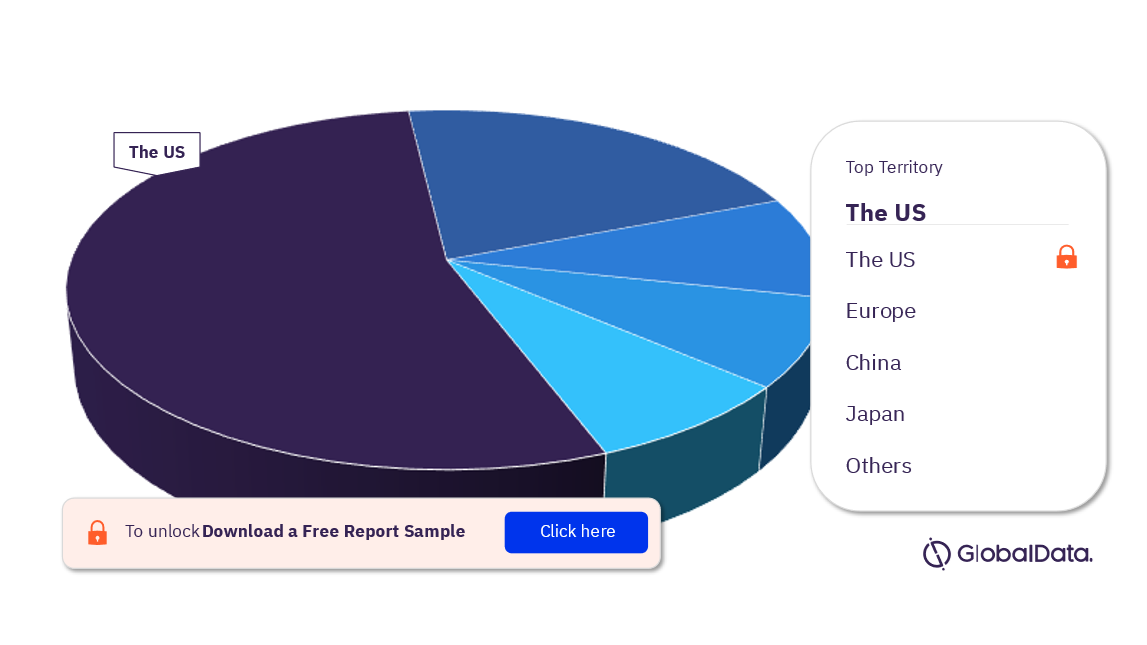
Interventional Neuroradiology – Pipeline Products by Territory 33
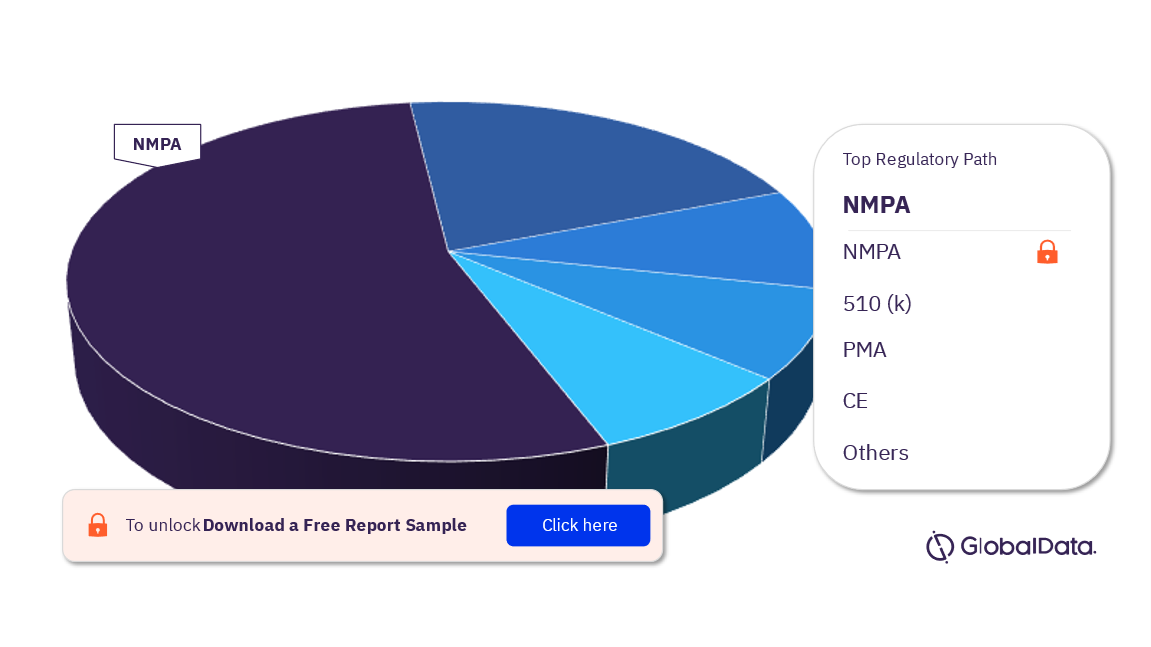
Interventional Neuroradiology – Pipeline Products by Regulatory Path 35
Interventional Neuroradiology – Pipeline Products by Estimated Approval Date 36
Interventional Neuroradiology – Ongoing Clinical Trials 37
Interventional Neuroradiology Companies – Pipeline Products by Stage of Development 38
Interventional Neuroradiology – Pipeline Products by Stage of Development 44
AccuMedical Medical Device (Beijing) Ltd Pipeline Products & Ongoing Clinical Trials Overview 52
Auxiliary Access Device – Product Status 52
Auxiliary Access Device – Product Description 52
Hemorrhagic Stroke Management Device – Product Status 53
Hemorrhagic Stroke Management Device – Product Description 53
Ischemic Stroke Management Device – Product Status 53
Ischemic Stroke Management Device – Product Description 53
Achieva Medical (Shanghai) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 54
Heat-fusion Detachable Coil – Product Status 54
Heat-fusion Detachable Coil – Product Description 54
Intermediate Catheter – Product Status 55
Intermediate Catheter – Product Description 55
Microcatheter – Product Status 55
Microcatheter – Product Description 55
MicroGuidewire – Product Status 56
MicroGuidewire – Product Description 56
NeuroStellar Intracranial Stent – Product Status 56
NeuroStellar Intracranial Stent – Product Description 57
SacEase Balloon Microcatheter – Product Status 57
SacEase Balloon Microcatheter – Product Description 57
Achieva Medical (Shanghai) Co Ltd – Ongoing Clinical Trials Overview 58
NeuroStellar Intracranial Stent – A Prospective, Multicenter, Single-arm Objective Performance Criteria Clinical Trial to Evaluate the Safety and Efficacy of Neurostellar Intracranial Stents in Patients with Symptomatic Intracranial Artery Stenosis 59
Acotec Scientific Holdings Ltd Pipeline Products & Ongoing Clinical Trials Overview 60
AcoArt Daisy – Product Status 60
AcoArt Daisy – Product Description 60
Carotid Stent – Product Status 61
Carotid Stent – Product Description 61
Intracranial PTA Balloon – Product Status 61
Intracranial PTA Balloon – Product Description 62
Peripheral Scoring Balloon – Product Status 62
Peripheral Scoring Balloon – Product Description 62
Peripheral Triple-Guidewire Balloon – Product Status 63
Peripheral Triple-Guidewire Balloon – Product Description 63
Actuated Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 64
NeuralGlider Insertion System – Product Status 64
NeuralGlider Insertion System – Product Description 64
Alcyone Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview 65
Alcyone Microcatheter – Product Status 65
Alcyone Microcatheter – Product Description 65
Amnis Therapeutics Ltd Pipeline Products & Ongoing Clinical Trials Overview 66
Golden Retriever – Product Status 66
Golden Retriever – Product Description 66
Anaconda BioMed SL Pipeline Products & Ongoing Clinical Trials Overview 67
ANA 5F Advanced Neurovascular Access – Product Status 67
ANA 5F Advanced Neurovascular Access – Product Description 67
ANCD BRAIN – Product Status 68
ANCD BRAIN – Product Description 68
Anaconda BioMed SL – Ongoing Clinical Trials Overview 69
ANA 5F Advanced Neurovascular Access – Prospective, Single-arm, Multi-center Study to Confirm the Safety and Performance of the ANA 5F Advanced Neurovascular Access, in Combination With a Stent Retriever in Patients With Acute Ischemic Stroke (ANAIS) 70
Ancure LLC Pipeline Products & Ongoing Clinical Trials Overview 71
Drug-Eluting Embolization Coil – Product Status 71
Drug-Eluting Embolization Coil – Product Description 71
Aneuclose LLC Pipeline Products & Ongoing Clinical Trials Overview 72
Janjua Aneurysm Net – Product Status 72
Janjua Aneurysm Net – Product Description 72
Janjua Aneurysm Occlusion Saddle – Product Status 73
Janjua Aneurysm Occlusion Saddle – Product Description 73
Aneuvas Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 74
PPODA-QT – Product Status 74
PPODA-QT – Product Description 74
Apeliotus Technologies Pipeline Products & Ongoing Clinical Trials Overview 75
Polymer Stent for Cerebral Aneurysms – Product Status 75
Polymer Stent for Cerebral Aneurysms – Product Description 75
Arterain Medical Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 76
Catheter Based System – Product Status 76
Catheter Based System – Product Description 76
Artio Medical Pipeline Products & Ongoing Clinical Trials Overview 77
Ballstent Microcatheter – Product Status 77
Ballstent Microcatheter – Product Description 77
Endura Embolization Coils – Product Status 78
Endura Embolization Coils – Product Description 78
Endura Embolization Device – Product Status 78
Endura Embolization Device – Product Description 79
Artiria Medical SA Pipeline Products & Ongoing Clinical Trials Overview 80
Smartguide Deflectable Hydrophilic Guidewire – Product Status 80
Smartguide Deflectable Hydrophilic Guidewire – Product Description 80
VascuSAFE – Product Status 81
VascuSAFE – Product Description 81
Artiria Medical SA – Ongoing Clinical Trials Overview 82
Smartguide Deflectable Hydrophilic Guidewire – Real-time Deflectable Guidewire in Neuro-interventions Study 83
Avantec Vascular Corp Pipeline Products & Ongoing Clinical Trials Overview 84
Endovascular Embolization Device – Product Status 84
Endovascular Embolization Device – Product Description 84
Ischemic Stroke Therapy Device – Product Status 85
Ischemic Stroke Therapy Device – Product Description 85
Balt USA LLC Pipeline Products & Ongoing Clinical Trials Overview 86
Squid Liquid Embolic Device – Product Status 86
Squid Liquid Embolic Device – Product Description 86
Balt USA LLC – Ongoing Clinical Trials Overview 87
Squid Liquid Embolic Device – The Squid Trial for the Embolization of the Middle Meningeal Artery (STEM) 88
BaseCamp Vascular Pipeline Products & Ongoing Clinical Trials Overview 89
GECKO Guidewire – Product Status 89
GECKO Guidewire – Product Description 89
Mono-Curve – Product Status 90
Mono-Curve – Product Description 90
Multi-Curve – Product Status 90
Multi-Curve – Product Description 90
Beijing Honghai Minimally Invasive Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 91
Cerebral Vein Thrombectomy Stent – Product Status 91
Cerebral Vein Thrombectomy Stent – Product Description 91
Intracranial Vein Stent – Product Status 92
Intracranial Vein Stent – Product Description 92
Bendit Technologies Ltd Pipeline Products & Ongoing Clinical Trials Overview 93
Bendit Microcatheter – Interventional Neuroradiology – Product Status 93
Bendit Microcatheter – Interventional Neuroradiology – Product Description 94
BioCure Inc Pipeline Products & Ongoing Clinical Trials Overview 95
GelCoil – Product Status 95
GelCoil – Product Description 95
LiquiGel – Product Status 96
LiquiGel – Product Description 96
Biomedical Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview 97
Tron Delta – Product Status 97
Tron Delta – Product Description 97
Boston University School of Medicine Pipeline Products & Ongoing Clinical Trials Overview 98
Balloon Guided Catheter – Product Status 98
Balloon Guided Catheter – Product Description 98
Capital Medical University Pipeline Products & Ongoing Clinical Trials Overview 99
Catfish Stent Retriever – Product Status 99
Catfish Stent Retriever – Product Description 99
Cerenovus Pipeline Products & Ongoing Clinical Trials Overview 100
Covered Stent – Product Status 100
Covered Stent – Product Description 100
Next Generation Balloon Expandable Stent – Product Status 101
Next Generation Balloon Expandable Stent – Product Description 101
Self-Expanding Stent – Product Status 101
Self-Expanding Stent – Product Description 102
Ceretrieve Ltd Pipeline Products & Ongoing Clinical Trials Overview 103
CathTrap – Product Status 103
CathTrap – Product Description 103
Cerovations LLC Pipeline Products & Ongoing Clinical Trials Overview 104
Reductive Ventricular Osmotherapy (RVOT) Catheter – Product Status 104
Reductive Ventricular Osmotherapy (RVOT) Catheter – Product Description 104
Cerus Endovascular Ltd Pipeline Products & Ongoing Clinical Trials Overview 105
Contour Neurovascular System – Product Status 105
Contour Neurovascular System – Product Description 105
Neqstent Coil Assisted Flow Diverter Device – Product Status 106
Neqstent Coil Assisted Flow Diverter Device – Product Description 106
Cerus Endovascular Ltd – Ongoing Clinical Trials Overview 107
Contour Neurovascular System – Intrasaccular Neck Occlusion Device Treatment of Intracranial Aneurysm in Acute Subarachnoid Hemorrhage 108
Contour Neurovascular System – US IDE Study of the Contour Neurovascular System for Intracranial Aneurysm Repair 108
Neqstent Coil Assisted Flow Diverter Device – Coil Assisted Flow Diversion: A Prospective, Single Arm, Multi-center Study to Assess the Safety and Performance of Neqstent in Adjunctive Therapy (CAFI Study) 109
Neqstent Coil Assisted Flow Diverter Device – Intrasaccular Neck Occlusion Device Treatment of Intracranial Aneurysm in Acute Subarachnoid Hemorrhage 109
Codman & Shurtleff Inc Pipeline Products & Ongoing Clinical Trials Overview 110
Occlusion Balloon – Product Status 110
Occlusion Balloon – Product Description 110
TRUFILL n-BCA Liquid Embolic System – Chronic Subdural Hematoma – Product Status 111
TRUFILL n-BCA Liquid Embolic System – Chronic Subdural Hematoma – Product Description 111
Codman & Shurtleff Inc – Ongoing Clinical Trials Overview 112
TRUFILL n-BCA Liquid Embolic System – Chronic Subdural Hematoma – Middle Meningeal Artery Embolization for the Treatment of Subdural Hematomas with TRUFILL n-BCA 113
Collagen Matrix Inc Pipeline Products & Ongoing Clinical Trials Overview 114
Collagen-based Coils – Product Status 114
Collagen-based Coils – Product Description 114
Conway Medical LLC Pipeline Products & Ongoing Clinical Trials Overview 115
Wrist-to-Brain Thrombectomy System – Product Status 115
Wrist-to-Brain Thrombectomy System – Product Description 115
Corindus Vascular Robotics Inc Pipeline Products & Ongoing Clinical Trials Overview 116
CorPath GRX Neurovascular System – Product Status 116
CorPath GRX Neurovascular System – Product Description 116
CranioVation Pipeline Products & Ongoing Clinical Trials Overview 117
Brain Aneurysm Clip – Product Status 117
Brain Aneurysm Clip – Product Description 117
Critical Innovations LLC Pipeline Products & Ongoing Clinical Trials Overview 118
BurRapid – Product Status 118
BurRapid – Product Description 118
CVTec Cerebrovascular Technologies GmbH Pipeline Products & Ongoing Clinical Trials Overview 119
CoilAlarm – Product Status 119
CoilAlarm – Product Description 119
Dartmouth College Pipeline Products & Ongoing Clinical Trials Overview 120
Aneurysm Clip Applicator – Product Status 120
Aneurysm Clip Applicator – Product Description 120
Cerebral Aneurysm Clip – Product Status 121
Cerebral Aneurysm Clip – Product Description 121
DePuy Synthes Inc Pipeline Products & Ongoing Clinical Trials Overview 122
CASHMERE Microcoil – Product Status 122
CASHMERE Microcoil – Product Description 122
Liquid Embolic System – Product Status 123
Liquid Embolic System – Product Description 123
MicruSphere Microcoil – Product Status 123
MicruSphere Microcoil – Product Description 124
Dongguan TT Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 125
All-in-One Neuroembolic Retriever – Product Status 125
All-in-One Neuroembolic Retriever – Product Description 125
BGC Neuroballoon Guiding Catheter – Product Status 126
BGC Neuroballoon Guiding Catheter – Product Description 126
NDB Nerve Dilatation Balloon Catheter – Product Status 126
NDB Nerve Dilatation Balloon Catheter – Product Description 127
EchoGuide Medical, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 128
Ultrasound Catheter Navigation Device – Product Status 128
Ultrasound Catheter Navigation Device – Product Description 128
Echopoint Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 129
iKOs Guidewire – Neuro Intervention – Product Status 129
iKOs Guidewire – Neuro Intervention – Product Description 129
Ecole Polytechnique Federale de Lausanne Pipeline Products & Ongoing Clinical Trials Overview 130
e-Dura Implant – Product Status 130
e-Dura Implant – Product Description 130
Embolx, Inc. Pipeline Products & Ongoing Clinical Trials Overview 131
Soldier High Flow Microcatheter – Product Status 131
Soldier High Flow Microcatheter – Product Description 131
Endomimetics LLC Pipeline Products & Ongoing Clinical Trials Overview 132
Blood Flow Diverter – Product Status 132
Blood Flow Diverter – Product Description 132
Brain Aneurysm Stent – Product Status 133
Brain Aneurysm Stent – Product Description 133
EndoShape, Inc. Pipeline Products & Ongoing Clinical Trials Overview 134
Next Generation Neurovascular Device – Radiopaque SMP – Product Status 134
Next Generation Neurovascular Device – Radiopaque SMP – Product Description 134
EndoStream Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview 135
Nautilus Intrasaccular System – Product Status 135
Nautilus Intrasaccular System – Product Description 135
EndoStream Medical Ltd – Ongoing Clinical Trials Overview 136
Nautilus Intrasaccular System – A Study to Evaluate the Nautilus Intrasaccular System to Treat Cerebral Aneurysms 137
Nautilus Intrasaccular System – Nautilus Endovascular Device for Wide Neck Cerebral Aneurysm Embolization Study 137
Euphrates Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview 138
Pulse MED System – Product Status 138
Pulse MED System – Product Description 138
Evasc Medical Systems Corp Pipeline Products & Ongoing Clinical Trials Overview 139
eCLIPs System – Product Status 139
eCLIPs System – Product Description 139
Second Generation eCLIPs System – Product Status 140
Second Generation eCLIPs System – Product Description 140
Evasc Medical Systems Corp – Ongoing Clinical Trials Overview 141
eCLIPs System – A Multicentre Post Marketing Study in France Evaluating the Safety and Efficacy of the eCLIPs Family of Products for the Treatment of Bifurcation Intracranial Aneurysms at the Carotid and Basilar Terminus 142
Fluid Biotech Inc Pipeline Products & Ongoing Clinical Trials Overview 143
Flow-Diverting Brain Stent – Product Status 143
Flow-Diverting Brain Stent – Product Description 143
Fluidx Medical Technology LLC Pipeline Products & Ongoing Clinical Trials Overview 144
GPX Embolic Device – Product Status 144
GPX Embolic Device – Product Description 144
Gaudi Vascular, Inc. Pipeline Products & Ongoing Clinical Trials Overview 145
Intracranial Stent – Product Status 145
Intracranial Stent – Product Description 145
Gentuity LLC Pipeline Products & Ongoing Clinical Trials Overview 146
Vis-M – Product Status 146
Vis-M – Product Description 146
Guichuangtongqiao Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 147
Blood Flow Diversion Device – Product Status 147
Blood Flow Diversion Device – Product Description 147
Intracranial Aneurysm Embolization Coil – Product Status 148
Intracranial Aneurysm Embolization Coil – Product Description 148
Hangzhou Yike Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 149
Neurointervention Device – Hemorrhagic Stroke – Product Status 149
Neurointervention Device – Hemorrhagic Stroke – Product Description 149
Neurointervention Device – Ischemic Stroke – Product Status 150
Neurointervention Device – Ischemic Stroke – Product Description 150
Neurointervention Device – Vascular Access – Product Status 150
Neurointervention Device – Vascular Access – Product Description 151
Hansen Medical Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 152
Magellan Robotic System – Neurological Procedures – Product Status 152
Magellan Robotic System – Neurological Procedures – Product Description 152
Hemo (China) Bioengineering Ltd Pipeline Products & Ongoing Clinical Trials Overview 153
Rapamycin Drug-Coated Intracranial Balloon Dilatation Catheter – Product Status 153
Rapamycin Drug-Coated Intracranial Balloon Dilatation Catheter – Product Description 153
Hemo (China) Bioengineering Ltd – Ongoing Clinical Trials Overview 154
Rapamycin Drug-Coated Intracranial Balloon Dilatation Catheter – A Prospective, Multicenter, Randomized Controlled, Best-benefit Clinical Trial to Evaluate the Safety and Efficacy of Rapamycin Drug-coated Intracranial Balloon Dilator Catheters for Intravascular Treatment of Symptomatic Intracranial Atherosclerotic Stenosis 155
Rapamycin Drug-Coated Intracranial Balloon Dilatation Catheter – Prospective, Multicenter, Single-group, Targeted Clinical Trial to Evaluate the Safety and Efficacy of Rapamycin Drug-coated Intracranial Balloon Dilatation Catheter for Intravascular Treatment of Symptomatic Intracranial Atherosclerotic Stenosis 155
HeMo Bioengineering Ltd Pipeline Products & Ongoing Clinical Trials Overview 156
Afentta Aspiration Catheter – Product Status 156
Afentta Aspiration Catheter – Product Description 157
Balloon Guiding Catheter – Product Status 157
Balloon Guiding Catheter – Product Description 157
Balloon-Expandable Stent – Product Status 158
Balloon-Expandable Stent – Product Description 158
Flow Diverter – Hemorrhagic Stroke – Product Status 158
Flow Diverter – Hemorrhagic Stroke – Product Description 159
Guiding Catheter – Product Status 159
Guiding Catheter – Product Description 159
HMC1-NAS Aspiration Catheter – Product Status 160
HMC1-NAS Aspiration Catheter – Product Description 160
HMSR-NAC Access Catheter – Product Status 161
HMSR-NAC Access Catheter – Product Description 161
HMSR-NAS Aspiration Catheter – Product Status 162
HMSR-NAS Aspiration Catheter – Product Description 162
Micro Guidewire – Ischemia – Product Status 162
Micro Guidewire – Ischemia – Product Description 163
Neuro Balloon Catheter – OTW – Product Status 163
Neuro Balloon Catheter – OTW – Product Description 163
Neuro Drug Eluting Stent – Product Status 164
Neuro Drug Eluting Stent – Product Description 164
Neuro Drug-Coated Balloon – Product Status 164
Neuro Drug-Coated Balloon – Product Description 165
Stent Retriever – Product Status 165
Stent Retriever – Product Description 165
Thrombectomy Stent – Product Status 166
Thrombectomy Stent – Product Description 166
HeMo Bioengineering Ltd – Ongoing Clinical Trials Overview 167
Neuro Drug Eluting Stent – To Evaluate a Prospective, Multicenter, Single-group, Target-value Clinical Trial of Rapamycin Drug-coated Intracranial Stents for the Intravascular Treatment of Symptomatic Intracranial Atherosclerotic Stenosis 168
Hibernia Medical Pipeline Products & Ongoing Clinical Trials Overview 169
Hybernia Safety Catheter – Neuroprotection – Product Status 169
Hybernia Safety Catheter – Neuroprotection – Product Description 169
HoloSurgical Inc Pipeline Products & Ongoing Clinical Trials Overview 170
ARAI Surgical Navigation System – Neurosurgery – Product Status 170
ARAI Surgical Navigation System – Neurosurgery – Product Description 170
Imperative Care Inc Pipeline Products & Ongoing Clinical Trials Overview 171
ZOOM 88 Large Distal Platform – Ischemic Stroke – Product Status 171
ZOOM 88 Large Distal Platform – Ischemic Stroke – Product Description 171
Imperative Care Inc – Ongoing Clinical Trials Overview 172
ZOOM 88 Large Distal Platform – Ischemic Stroke – A Prospective, Multi-center, Open Label and Single Arm Clinical Investigation to Evaluate the Safety and Efficacy of Using the Zoom Reperfusion System in Thrombectomy Procedures to Treat Acute Ischemic Stroke Patients 173
Inretio Ltd Pipeline Products & Ongoing Clinical Trials Overview 174
PREVA – Product Status 174
PREVA – Product Description 174
Insera Therapeutics LLC Pipeline Products & Ongoing Clinical Trials Overview 175
CLEAR Aspiration System – Product Status 175
CLEAR Aspiration System – Product Description 175
Shelter Retriever – Product Status 176
Shelter Retriever – Product Description 176
InspireMD Inc Pipeline Products & Ongoing Clinical Trials Overview 177
NGuard – Product Status 177
NGuard – Product Description 177
Integra LifeSciences Holdings Corp Pipeline Products & Ongoing Clinical Trials Overview 178
Deltapaq Microcoil – Product Status 178
Deltapaq Microcoil – Product Description 179
Flow Diverter – Product Status 179
Flow Diverter – Product Description 179
Irras AB Pipeline Products & Ongoing Clinical Trials Overview 180
Hummingbird – Cranial Access Kit – Product Status 180
Hummingbird – Cranial Access Kit – Product Description 180
iVascular SLU Pipeline Products & Ongoing Clinical Trials Overview 181
Interventional Neuroradiology – Catheter – Product Status 181
Interventional Neuroradiology – Catheter – Product Description 181
Jiangsu Nuanyang Medical Instrument Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 182
YonFlow Blood Flow Steering Stent System – Product Status 182
YonFlow Blood Flow Steering Stent System – Product Description 182
Jiangsu Nuanyang Medical Instrument Co Ltd – Ongoing Clinical Trials Overview 183
YonFlow Blood Flow Steering Stent System – Study on the Clinical Safety and Effectiveness of Blood Flow Guided Stent System for Intracranial Aneurysms 184
Juhui Medical Technology (Shenzhen) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 185
J-Tube – Product Status 185
J-Tube – Product Description 185
Juhui Medical Technology (Shenzhen) Co Ltd – Ongoing Clinical Trials Overview 186
J-Tube – Prospective, Multicenter, Single-group, Target-value Clinical Trial to Evaluate the Efficacy and Safety of J-tube in the Treatment of Intracranial Aneurysms 187
Liaoning Yinyi Biotechnology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 188
Vmoky – Product Status 188
Vmoky – Product Description 188
Liaoning Yinyi Biotechnology Co Ltd – Ongoing Clinical Trials Overview 189
Vmoky – Evaluation of the Safety and Efficacy of Drug Eluting Balloon Catheter for the Treatment of Patients with Symptomatic Intracranial Atherosclerosis Stenosis 190
LuSeed Vascular Pipeline Products & Ongoing Clinical Trials Overview 191
LuSeed Intrasaccular Device – Cerebral Aneurysm – Product Status 191
LuSeed Intrasaccular Device – Cerebral Aneurysm – Product Description 191
Mayo Clinic Pipeline Products & Ongoing Clinical Trials Overview 192
Tissue-Derived Bioactive Gel – Product Status 192
Tissue-Derived Bioactive Gel – Product Description 192
Medikit Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 193
Aspirarecath – Product Status 193
Aspirarecath – Product Description 194
Axcelguide – Product Status 194
Axcelguide – Product Description 194
Cerulean G – Product Status 195
Cerulean G – Product Description 195
Slimguide – Product Status 195
Slimguide – Product Description 196
Medtronic Plc Pipeline Products & Ongoing Clinical Trials Overview 197
Artisse Intrasaccular Device – Product Status 197
Artisse Intrasaccular Device – Product Description 198
Intrasaccular Device – Hemorrhagic Stroke – Product Status 198
Intrasaccular Device – Hemorrhagic Stroke – Product Description 198
Lazarus Locket – Product Status 199
Lazarus Locket – Product Description 199
MindFrame IRIIS System – Product Status 199
MindFrame IRIIS System – Product Description 200
Onyx Liquid Embolic System – Middle Meningeal Arterial Embolization – Product Status 200
Onyx Liquid Embolic System – Middle Meningeal Arterial Embolization – Product Description 200
Medtronic Plc – Ongoing Clinical Trials Overview 201
Artisse Intrasaccular Device – ARTISSE Aneurysm Treatment Using Intrasaccular Flow Diversion with the ARTISSE Device 202
Onyx Liquid Embolic System – Middle Meningeal Arterial Embolization – Managing Non-acute Subdural Hematoma Using Liquid Materials: A Chinese Randomized Trial of MMA Treatment 203
Onyx Liquid Embolic System – Middle Meningeal Arterial Embolization – The Onyx Trial for the Embolization of the Middle Meningeal Artery for Chronic Subdural Hematoma (OTEMACS) 203
Merlin MD Pte. Ltd. Pipeline Products & Ongoing Clinical Trials Overview 204
Next Generation Device – Intracranial Aneurysm – Product Status 204
Next Generation Device – Intracranial Aneurysm – Product Description 204
MicroPort NeuroTech Ltd Pipeline Products & Ongoing Clinical Trials Overview 205
Blumen Intracranial Visualized Stent System – Product Status 205
Blumen Intracranial Visualized Stent System – Product Description 206
Clot Retrieval Device – Product Status 206
Clot Retrieval Device – Product Description 206
Embolization Coil – Product Status 207
Embolization Coil – Product Description 207
Intracranial Drug-Coated Balloon Catheter System – Product Status 207
Intracranial Drug-Coated Balloon Catheter System – Product Description 208
Neuro-Guidewire – Product Status 208
Neuro-Guidewire – Product Description 208
Tubridge Plus Flowdiverting Stent – Product Status 209
Tubridge Plus Flowdiverting Stent – Product Description 209
Tubridge-II Vascular Reconstruction Device – Product Status 209
Tubridge-II Vascular Reconstruction Device – Product Description 210
W-track Intracranial Aspiration Catheter – Product Status 210
W-track Intracranial Aspiration Catheter – Product Description 210
MicroPort Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview 211
Balloon Protection Guiding Catheter – Product Status 211
Balloon Protection Guiding Catheter – Product Description 211
Micro-Catheter – Product Status 212
Micro-Catheter – Product Description 212
Rebridge Intracranial Visualized Stent – Product Status 212
Rebridge Intracranial Visualized Stent – Product Description 213
MicroPort Scientific Corp – Ongoing Clinical Trials Overview 214
Rebridge Intracranial Visualized Stent – Prospective, Multi-center, Open-label, Non-inferiority, Randomized, Controlled Registration Trial of the Intracranial Visualized Stent for the Wide-necked Intracranial Aneurysms 215
MicroVention Inc Pipeline Products & Ongoing Clinical Trials Overview 216
Advanced Coil – Product Status 216
Advanced Coil – Product Description 216
Coated Stent – Product Status 217
Coated Stent – Product Description 217
Minor MicroPort Pipeline Products & Ongoing Clinical Trials Overview 218
Balloon Catheter – Product Status 218
Balloon Catheter – Product Description 218
Cerebral Hemorrhage Coil – Product Status 219
Cerebral Hemorrhage Coil – Product Description 219
Cerebrovascular Stent – Product Status 219
Cerebrovascular Stent – Product Description 219
Dense Mesh Stent – Product Status 220
Dense Mesh Stent – Product Description 220
Thrombectomy Device – Product Status 220
Thrombectomy Device – Product Description 221
MiVi Neuroscience LLC Pipeline Products & Ongoing Clinical Trials Overview 222
DAISe Clot Retrieval System – Product Status 222
DAISe Clot Retrieval System – Product Description 222
MIVI Q Revascularization System – Product Status 223
MIVI Q Revascularization System – Product Description 223
MiVi Neuroscience LLC – Ongoing Clinical Trials Overview 224
MIVI Q Revascularization System – A Prospective, Multi-center, Single Arm Study to Evaluate the Q Revascularization System for Neurointervention in Acute Ischemic Stroke: The EvaQ Study 225
DAISe Clot Retrieval System – A Feasibility Study for the DAISe Thrombectomy Device in Colombia 226
DAISe Clot Retrieval System – A Prospective, Multi-center, Single Arm, Feasibility Study to Evaluate the DAISe Thrombectomy System During Neurointervention for Acute Ischemic Stroke; DAISe 1 Study 226
Monarch Biosciences Inc Pipeline Products & Ongoing Clinical Trials Overview 227
Titan Flow Diverter – Product Status 227
Titan Flow Diverter – Product Description 227
Nanjing Kanite Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview 228
Stent Retriever – Stroke – Product Status 228
Stent Retriever – Stroke – Product Description 228
Nanjing SealMed Medical Technology Corp Ltd Pipeline Products & Ongoing Clinical Trials Overview 229
Embolization Assisting Balloon – Product Status 229
Embolization Assisting Balloon – Product Description 229
Navisonics Inc Pipeline Products & Ongoing Clinical Trials Overview 230
NaviSonics Surgical Navigation System – Product Status 230
NaviSonics Surgical Navigation System – Product Description 230
NEOS Surgery SL Pipeline Products & Ongoing Clinical Trials Overview 231
Aneurysm Clip – Product Status 231
Aneurysm Clip – Product Description 231
Biomedical Implant – Intracranial Aneurysm – Product Status 232
Biomedical Implant – Intracranial Aneurysm – Product Description 232
Neurogami Medical Inc Pipeline Products & Ongoing Clinical Trials Overview 233
Gen2 ThromboTube – Product Status 233
Gen2 ThromboTube – Product Description 233
Intra-Aneurysmal Rapid Occlusion Device – Product Status 234
Intra-Aneurysmal Rapid Occlusion Device – Product Description 234
NeuroInterventional Therapeutics, Inc. Pipeline Products & Ongoing Clinical Trials Overview 235
NIT Ischemic Stroke Intervention System – Product Status 235
NIT Ischemic Stroke Intervention System – Product Description 235
Traumatic Brain Injury Catheter – Product Status 236
Traumatic Brain Injury Catheter – Product Description 236
Neurosigma Inc Pipeline Products & Ongoing Clinical Trials Overview 237
DELTA Stent – Product Status 237
DELTA Stent – Product Description 237
Thin Film Nitinol Flow Diverting Stent – Product Status 238
Thin Film Nitinol Flow Diverting Stent – Product Description 238
Neurosyntec LLC. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 239
Neuro-Interventional Catheter System – Product Status 239
Neuro-Interventional Catheter System – Product Description 239
NeuroVasc Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview 240
eNVac Aspiration Catheter – Product Status 240
eNVac Aspiration Catheter – Product Description 240
Envi-SR – Product Status 241
Envi-SR – Product Description 241
eNVoke Intermediate Catheter – Product Status 241
eNVoke Intermediate Catheter – Product Description 242
eNVoke Microcatheter – Product Status 242
eNVoke Microcatheter – Product Description 242
Versi System – Product Status 243
Versi System – Product Description 243
NeuroVasc Technologies Inc – Ongoing Clinical Trials Overview 244
Versi System – Multi Center, Prospective, Registry Trial of Versi Retriever Mechanical Thrombecomy for Acute Ischemic Stroke in Japan 245
Envi-SR – A Chinese Pivotal Randomized Controlled Trial of ENVI-SR Mechanical Thrombectomy System in Patients with Acute Ischemic Stroke 246
Envi-SR – A Randomized Controlled Trial of Envi-SR for Endovascular Treatment of Ischemic Stroke: US IDE Clinical Trial 246
NexGen Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview 247
EViTAR MulticoilMR – Product Status 247
EViTAR MulticoilMR – Product Description 247
North Carolina State University Pipeline Products & Ongoing Clinical Trials Overview 248
Vortex Ultrasound Transducer – Product Status 248
Vortex Ultrasound Transducer – Product Description 248
Novasentis Inc Pipeline Products & Ongoing Clinical Trials Overview 249
Electroactive Polymer based Catheter-Cranial – Product Status 249
Electroactive Polymer based Catheter-Cranial – Product Description 249
Occam Design LLC Pipeline Products & Ongoing Clinical Trials Overview 250
Porous Brain Infusion Catheter – Product Status 250
Porous Brain Infusion Catheter – Product Description 250
OccluTex Medical BV Pipeline Products & Ongoing Clinical Trials Overview 251
OccluTex – Product Status 251
OccluTex – Product Description 251
OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview 252
Flow Diverter Device – Product Status 252
Flow Diverter Device – Product Description 253
Neuro Aspiration Catheter – Product Status 253
Neuro Aspiration Catheter – Product Description 253
Neuro Balloon Catheter – Product Status 254
Neuro Balloon Catheter – Product Description 254
Neuro DEB Catheter – Product Status 254
Neuro DEB Catheter – Product Description 255
Neuro Distal Protection Device – Product Status 255
Neuro Distal Protection Device – Product Description 255
Neuro Microcatheter – Product Status 256
Neuro Microcatheter – Product Description 256
Neuro Occulsion Balloon Catheter – Product Status 256
Neuro Occulsion Balloon Catheter – Product Description 257
Neuro Retriver Device – Product Status 257
Neuro Retriver Device – Product Description 257
Oxford Endovascular Ltd Pipeline Products & Ongoing Clinical Trials Overview 258
Oxiflow – Product Status 258
Oxiflow – Product Description 258
Palmaz Scientific Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview 259
Micro Neuro-Stent – Product Status 259
Micro Neuro-Stent – Product Description 259
![]()