20 Oct, 2020 MenABCWY vaccines to begin climb to dominance, taking 21% share of 2029’s $3.17bn global meningococcal vaccine market
Posted in PharmaThe launch of pentavalent vaccines from GlaxoSmithKline and Pfizer that protect against the meningococcal serogroups A, B, C, W, and Y (MenABCWY) in the nine major markets (9MM*) are expected to reduce the vaccine burden and be more cost-effective for healthcare systems – thus driving growth in the meningococcal vaccine market over the next ten years, according to GlobalData, a leading data and analytics company.
GlobalData’s latest report, ‘Meningococcal Vaccines: Global Drug Forecast and Market Analysis to 2029’, notes that the size of the meningococcal vaccine market across the 9MM will increase from $3.07bn in 2019 to $3.17bn in 2029 at a modest compound annual growth rate (CAGR) of 0.3%. MenABCWY vaccines are expected to start to replace the current dominant vaccine types in this market to emerge with a share of 21% – with sales of $670m – by 2029.
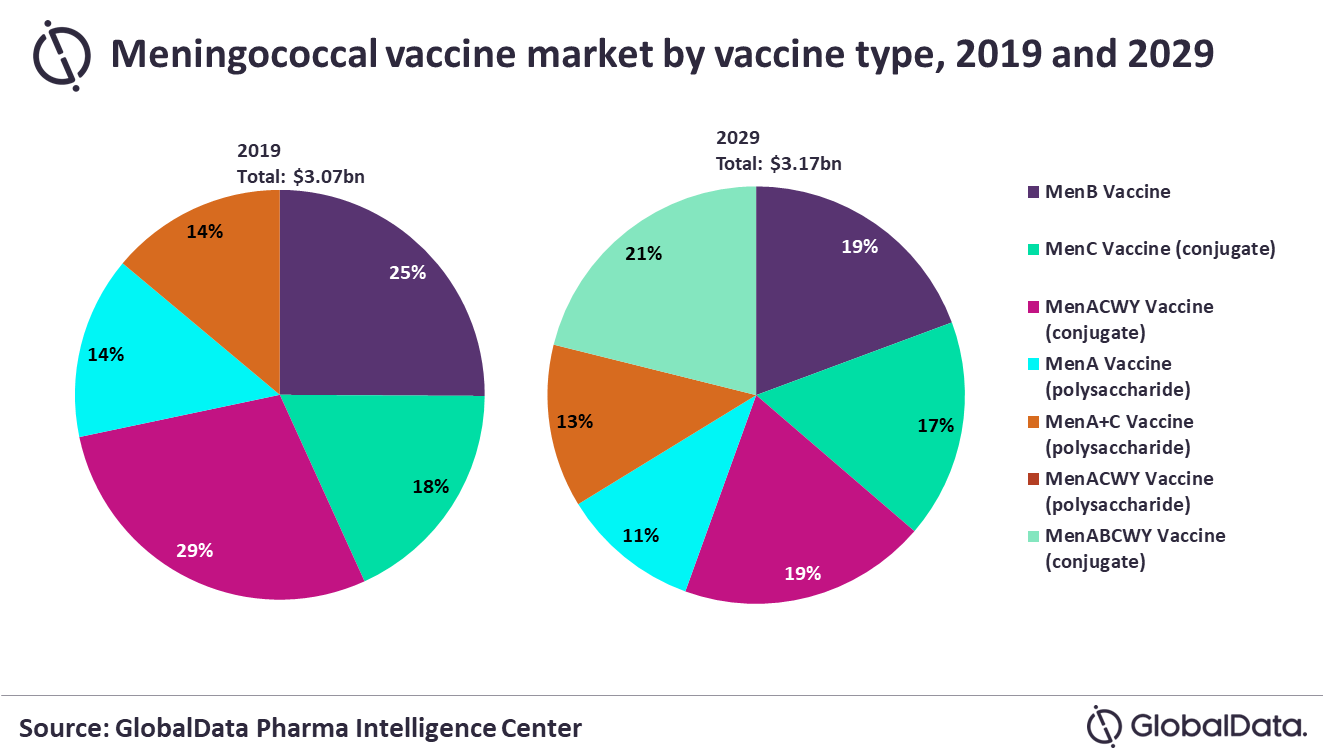
Philipp Rosenbaum, PhD, Pharma Analyst at GlobalData, comments: “This spells bad news for Sanofi, which is the leader in sales of vaccines protecting against serogroups A, C, W, and Y (MenACWY) in the US. Meanwhile, GSK and Pfizer, which both produce MenACWY and MenB vaccines, will gain market share with their new MenABCWY vaccines. MenACWY vaccines had a 29% share of the market in 2019 while those protecting against serogroup B (MenB) had 25%. While the spread of pentavalent MenABCWY vaccines to the US, 5EU and Brazil will be a major driver of growth in this submarket, uptake will be slow – especially in the 5EU and Brazil – due to slow implementation of new vaccine guidelines.
“Meningococcal vaccination recommendations differ widely between the 9MM. The market would ideally benefit from updates in countries such as France, Germany, Brazil and China to enhance uptake and provide better and broader protection against meningococcal disease for the most vulnerable populations.”
GSK’s and Pfizer’s MenABCWY vaccines will reach the highest sales in the US – since both vaccines are currently in development for the age group 10–25 years of age and both MenACWY and MenB vaccines are recommended for adolescents in this country. In the rest of the 9MM, the majority of meningococcal vaccine doses are recommended for infants, so pentavalent vaccines are likely being used initially only for adolescent booster doses in the UK, Italy and Spain.
Since meningococcal disease can be severe – but the incidence rate is very low – cost-effectiveness plays another major role in national immunization recommendations. Key Opinion Leaders (KOLs) interviewed by GlobalData stated that pentavalent vaccines would be welcomed to reduce the vaccine burden but will have to show good efficacy data and duration of protection.
*9MM = The US, Germany, France, Italy, Spain, the UK, Argentina, Brazil and China
*5EU = Germany, France, Italy, Spain and the UK
