16 Sep, 2019 Burgeoning oncology pipeline increases demand for containment manufacturing, good news for dedicated CMOs investing in containment facilities
Posted in Press ReleaseThe burgeoning oncology pipeline is particularly good news for AMRI, Apeloa, Cambrex, Lonza and Patheon which have multiple containment facilities, according to GlobalData, a leading data and analytics company.
There were a total of 1,754 small molecule active pharmaceutical ingredient (API) contract manufacturing facilities included in PharmSource’s API report’s analysis with 29% offering containment capability, and 7% offering controlled substance capability. Containment and controlled substance capabilities are specialty offerings and defining features. AMRI, Apeloa, and Patheon (owned by Thermo Fisher Scientific) are among the contract manufacturing organizations (CMOs) with the highest number of containment facilities able to handle substances such as antibiotics, high potency non-cytotoxics, cytotoxics, and steroids.
Demand for containment manufacturing will continue to increase in the future as the oncology drug development pipeline continues to grow.
The company’s latest report, ‘PharmSource – Contract Small Molecule API Manufacturing Industry by the Numbers – 2019 Edition’, characterizes the contract small molecule API manufacturing industry through a number of quantitative dimensions, including number and type of participants, containment and controlled substance capabilities. Contract small molecule API manufacturing is critical for establishing an understanding of the small molecule API CMO industry and features some of the largest CMOs participating in the industry.
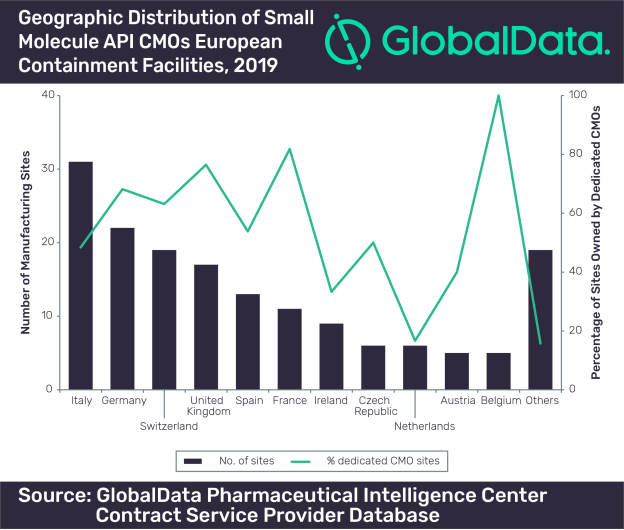
In terms of geographic distribution of small molecule API manufacturing facilities, China has the largest number, followed by India and then the US. 31% of US small molecule API facilities have containment capabilities compared with 26% of Chinese facilities and 20% of those in India.
Adam Bradbury, PharmSource Analyst at GlobalData, commented: “The emerging markets of China and India provide much of the small molecule API supply globally as well as lower-cost labor and fewer regulations than in North America. Europe makes Chinese and Indian facilities ideal for CMOs seeking small molecule API manufacture. The savings are passed onto the consumer and generics can be sold for cheaper in an already competitive environment”.
The US opioid abuse epidemic and the resulting lawsuits have made it a particularly hazardous time for those that market opioids; Insys Therapeutics and Purdue Pharma have both filed for bankruptcy. Dedicated CMOs flourish in controlled substances manufacture; however, a reduction in opioid production may negatively affect CMOs involved. AMRI, Ajinomoto, Cambrex, Johnson Matthey, and Patheon are among the contractors with the largest number of controlled substance facilities.
Bradbury continues: “Controlled substance capabilities and facility requirements can be prohibitively expensive. As such, only the largest CMOs can acquire, construct, and/or maintain a controlled substance facility. Therefore, any reaction in the US involving a reduction in their production will negatively affect the larger CMOs involved”.
Note: Only facility counts for companies engaged in contract manufacturing are included in this figure. Dedicated CMOs only perform contract manufacturing, not both manufacturing and marketing their own products.
Small molecule API mergers and acquisitions (M&A) activity involving CMOs between 2015 to 2018 further highlights the desirability of containment facilities – when 53% of facilities acquisitions by both dedicated and excess capacity CMOs were for facilities with containment.
Bradbury concludes: “The number of high-potency new molecular entity (NME) approvals has increased over the last decade, especially for oncology kinase inhibitors. This is a positive sign for CMOs as small and mid-cap companies lack the expertise for regulatory compliance and high containment facilities and seek to outsource NMEs requiring special handling”.
