
Pharma DECODED
Previous edition: 03 Apr 2024
Share article
Get the full version straight to your inbox.
Exclusive access to our best-in-class data & intelligence
Subscribe now
FDA accepts AstraZeneca-Daiichi Sankyo's Datopotamab deruxtecan BLA
The regulatory milestone is based on the outcomes of the TROPION-Breast01 Phase III clinical trial.

The US Food and Drug Administration (FDA) has accepted AstraZeneca and Daiichi Sankyo's biologics licence application (BLA) for datopotamab deruxtecan (Dato-DXd), a new treatment option for a subset of breast cancer patients.
The application sought approval for the use of Dato-DXd in adults with unresectable or metastatic hormone receptor (HR)-positive, HER2-negative (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer who have undergone previous systemic therapy.
The Prescription Drug User Fee Act deadline for the FDA's decision will be in the first quarter of 2025.
The regulatory milestone is based on the outcomes of the TROPION-Breast01 Phase III clinical trial, which is a global, randomised, multicentre, open-label study designed to assess the efficacy and safety of Dato-DXd against selected single-agent chemotherapies.
Dato-DXd showed an improvement in progression-free survival versus the investigator's choice of chemotherapy.
While interim results for the dual primary endpoint of overall survival indicated a numerical advantage for Dato-DXd over chemotherapy, the findings were not mature at the time of the data cut-off.
Dato-DXd's safety profile was in line with earlier studies.
Datopotamab deruxtecan is a TROP2-directed DXd antibody-drug conjugate initially discovered by Daiichi Sankyo. The asset is being developed in partnership with AstraZeneca under a global agreement signed in July 2020.
Another BLA for Dato-DXd is under review in the US, based on results from the pivotal TROPION-Lung01 Phase III trial for treating adult patients with locally advanced or metastatic nonsquamous non-small cell lung cancer post systemic therapy.
AstraZeneca oncology research and development executive vice-president Susan Galbraith stated: “Despite marked progress in the treatment of HR-positive, HER2-negative breast cancer, most patients with advanced disease develop endocrine resistance and face the prospect of one or several lines of chemotherapy.
“If approved, datopotamab deruxtecan has the potential to provide these patients an efficacious and better-tolerated alternative to conventional chemotherapy.”
Last month, the Japanese Ministry of Health, Labour and Welfare approved AstraZeneca‘s Truqap (capivasertib) plus Faslodex (fulvestrant) for a specific type of breast cancer.
Latest news

Genmab joins ADC arena with $1.8bn ProfoundBio acquisition
Genmab partakes in the flurry of financial activity being seen with the promising cancer therapeutic modality of antibody drug conjugates.

Ipsen joins the ADC club with deal worth up to $900m
Ipsen signed a licensing agreement to develop and commercialise Sutro Biopharma’s ADC STRO-003 intended as a treatment for solid tumours.

Bavarian Nordic expands access to mpox vaccine in US
Jynneos contributed $725m in sales to Bavarian Nordic in 2023, being a huge driver in revenues for the company.
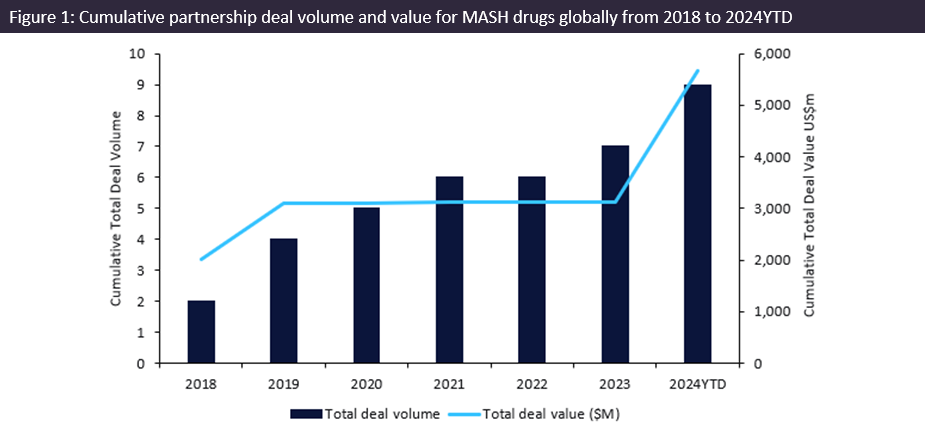
MASH revival with over $2.5bn for innovator drug partnership deals in 2024
MASH, previously known as nonalcoholic steatohepatitis (NASH), is a disease characterised by liver inflammation and damage caused by the accumulation of fat.

Roivant and Priovant report positive data from non-infectious uveitis trial
Regarding the primary endpoint of treatment failure, results showed lower rates, which indicates a greater treatment benefit.

Resonance Health agrees to acquire TrialsWest
Subject to meeting specific conditions, the deal is anticipated to conclude in the last quarter of FY 2024.

YolTech doses first subject in familial hypercholesterolemia trial
The trial will evaluate the preliminary impact of the drug on plasma lipid and lipoprotein levels.

13th Annual Clinical Trial Supply New England 2024
The upcoming Clinical Trial Supply Europe 2024 conference will gather pharma and biotech delegates to discuss the trends and challenges in the clinical trial supply industry.
In our previous edition

Pharma Decoded
Alexion's Voydeya gains FDA approval for extravascular haemolysis
02 Apr 2024

Pharma Decoded
Akebia's Vafseo gains FDA approval for anaemia due to CKD
28 Mar 2024

Pharma Decoded
Regeneron hit with FDA rejection for blood cancer therapy
27 Mar 2024
Newsletters in other sectors
Aerospace, Defence & Security
Automotive
Banking & Payments
Medical Devices
Travel and Tourism
Search companies, themes, reports, as well as actionable data & insights spanning 22 global industries
Access more premium companies when you subscribe to Explorer


