
Pharma DECODED
Previous edition: 16 May 2024
Share article
Get the full version straight to your inbox.
Exclusive access to our best-in-class data & intelligence
Subscribe now
FDA approves Bristol Myers Squibb's Breyanzi for follicular lymphoma
The approval is based on results from the open-label, global, multi-centre, single-arm Phase II TRANSCEND FL clinical trial.
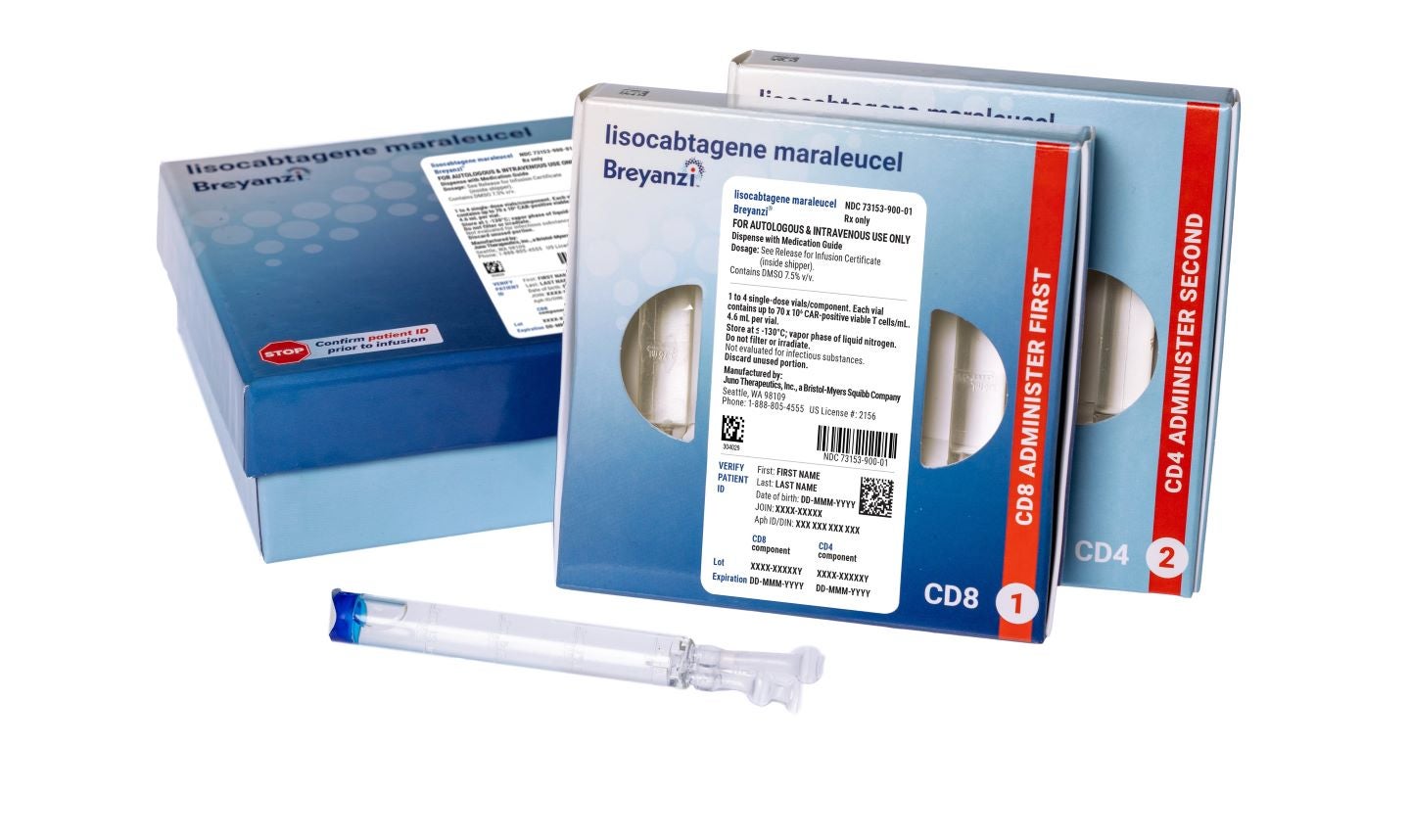
The US Food and Drug Administration (FDA) has granted accelerated approval for Bristol Myers Squibb’s Breyanzi (lisocabtagene maraleucel; liso-cel) for adult patients with relapsed or refractory follicular lymphoma (FL).
The CD19-directed chimeric antigen receptor (CAR) T cell therapy is indicated for patients who have undergone at least two previous systemic therapies.
Administered as a one-time infusion, Breyanzi delivers a dose of 90 to 110 × 10⁶ CAR-positive viable T cells to patients.
The approval is based on results from the open-label, global, multicentre, single-arm Phase II TRANSCEND FL clinical trial evaluating Breyanzi's efficacy and safety.
The primary outcome measure was the overall response rate while secondary measures included complete response rate, duration of response, progression-free survival and safety.
The trial demonstrated an overall response rate of 95.7% while the complete response rate was 73.4%.
Responses were reported to be quick, with a median time of one month, and durable. The median duration of response has not yet been reached.
At 12 and 18 months, the majority remained in response.
Breyanzi has shown a consistent safety profile throughout the trials.
Cytokine release syndrome (CRS) of any grade was reported in 53% of subjects with Grade >3 CRS seen in 4% of patients.
Bristol Myers Squibb cell therapy commercial head and senior vice-president Bryan Campbell stated: “Breyanzi is a cornerstone of our cell therapy portfolio, providing a differentiated profile across a wide array of B-cell malignancies.
“The approval of Breyanzi for relapsed or refractory FL provides an option with potential for lasting remission in a one-time infusion and a safety profile that allows for administration and monitoring in both the inpatient and outpatient setting in an increasing number of certified treatment centres in the US.”
Last month, the European Commission granted expanded approval for Bristol Myers Squibb‘s Reblozyl (luspatercept) as a first-line treatment for adults with transfusion-dependent anaemia associated with very low, low and intermediate-risk myelodysplastic syndromes.
Latest news

KRAS inhibitors: The next frontier beckons
There has been several developments in the KRAS inhibitor space, shaping the KRAS inhibitors of the future.

Takeda's dengue vaccine obtains WHO prequalification
The TAK-003 vaccine targets all four virus serotypes.

FDA rejects label expansion for Dynavax's hepatitis B vaccine
The US agency rejected the expanded use of Dynavax’s vaccine for adults on haemodialysis, citing insufficient efficacy and safety data.

Tracking the opioid lawsuit settlements amidst calls for oversight
The US National Opioid Settlement is distributing more than $54 billion to communities grappling with the opioid crisis.

Vanda Pharmaceuticals reports positive data from motion sickness trial
In the trial, only 10.4% and 18.3% of the subjects reported vomiting on the 170mg and 85mg doses, respectively.

Moleculin begins NIH-funded Phase II glioblastoma treatment trial
The primary goal of the study is to measure progression-free survival.
Allucent receives grant for decentralised Covid-19 vaccine trial
The company received the award to conduct a trial assessing correlates of protection following Covid-19 vaccination.
In our previous edition

Pharma Decoded
Circular RNA: Vaccines, therapeutics and biomarkers could be revolutionised
15 May 2024

Pharma Decoded
Sanofi to make €1bn biomanufacturing investment in France
14 May 2024

Pharma Decoded
RAPT terminates Phase II trials for lead candidate following clinical hold
13 May 2024
Newsletters in other sectors
Aerospace, Defence & Security
Automotive
Banking & Payments
Medical Devices
Oil & Gas
Travel and Tourism
Search companies, themes, reports, as well as actionable data & insights spanning 22 global industries
Access more premium companies when you subscribe to Explorer


